题目内容
(14分)
氢溴酸在工业和医药领域中有着广泛的用途,下图是某兴趣小组模拟工厂制备氢溴酸粗品并精制的流程如下:
(1)混合①使用冰水的目的是 ;
(2)操作II和III的名称是 , ;
(3)混合②中发生反应的离子方程式为 ;
(4)工业上用氢溴酸和大理石制得溴化钙中含有少量Al3+、Fe3+杂质,加入适量的试剂 (填化学式)后控制溶液的PH约为8.0即可除去杂质,控制溶液的PH约为8.0的目的是_______________________________________________________;
(5)t℃时,将HBr通入AgNO3溶液中生成的AgBr在水中的沉淀溶解平衡曲线如图所示,又知t℃时AgCl的Ksp=4×l0-10,下列说法不正确的是 ( )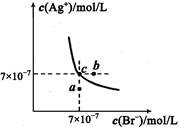
| A.向含有Cl-和Br- 的混合液中滴加硝酸银溶液,一定先产生AgBr的沉淀 |
| B.在AgBr饱和溶液中加入NaBr固体,可使溶液由c点到b点 |
| C.图中a点对应的是AgBr的不饱和溶液 |
D.在t℃时,AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)平衡常数Κ≈816 AgBr(s)+Cl-(aq)平衡常数Κ≈816 |
(每空2分)
(1)降低体系的温度,防止溴的挥发(2分)
(2)过滤,蒸馏(各2分)
(3) Br2+SO32-+H2O=2Br-+SO42-+2H+(2分)
(4) CaO或Ca(OH)2或CaCO3,确保Fe3+和Al3+沉淀完全和防止氢氧化铝溶解(各2分)
(5)AB(2分)
解析试题分析:(1)Br2氧化SO2放出很多热量,溴易挥发,使用冰水,降低体系温度,防止溴蒸发,使反应完全。
(2)由工艺流程可知,操作Ⅱ分离固体与液体,应是过滤;操作Ⅲ为互溶的溶液组分的分离,应是蒸馏。
(3)混合②中加入Na2SO3,Na2SO3具有还原性,被Br2氧化,所以离子方程式为:Br2+SO32-+H2O=2Br-+SO42-+2H+
(4)目的是制取CaBr2,通过控制溶液的PH约为8.0即可除去杂质Al3+、Fe3+,为了防止新杂质的进入,应加入含Ca元素且能与H+反应的物质,如CaO或Ca(OH)2或CaCO3;控制溶液的PH约为8.0时,Al3+、Fe3+转为Al(OH)3和Fe(OH)3沉淀而除去,所以控制溶液的PH约为8.0的目的是确保Fe3+和Al3+沉淀完全和防止氢氧化铝溶解。
(5)A、根据图中c点坐标可求出AgBr的Ksp=7×10-7×7×10-7= 4.9×10-13,小于AgCl的Ksp,但选项中没有给出Cl?和Br?浓度,所以不一定先产生AgBr的沉淀,错误;B、在AgBr饱和溶液中加入NaBr固体,沉淀溶解平衡移动后溶液仍为饱和溶液,不可能由c点到b点,错误;C、a点在溶解平衡曲线以下,所以a点对应的是AgBr的不饱和溶液,正确;D、在t℃时,AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)平衡常数Κ=c(Cl?)/c(Br?)= c(Cl?)?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4×l0-10/4.9×10-13≈816,正确。
AgBr(s)+Cl-(aq)平衡常数Κ=c(Cl?)/c(Br?)= c(Cl?)?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4×l0-10/4.9×10-13≈816,正确。
考点:本题考查化学流程的分析、基本操作、除杂、沉淀溶解平衡。

 状元坊全程突破导练测系列答案
状元坊全程突破导练测系列答案常温下,将某一元碱BOH和HCl溶液等体积混合,两种溶液的浓度和混合后所得溶液的pH如下表:
| 实验编号 | HCl的物质的量浓度 (mol·L-1) | BOH的物质的量浓度 (mol·L-1) | 混合溶液的pH |
| ① | 0.1 | 0.1 | pH=5 |
| ② | c | 0.2 | pH=7 |
| ③ | 0.1 | 0.2 | pH>7 |
请回答:
(1)从第①组情况分析,BOH是 (选填“强碱”或“弱碱”)。该组所得混合溶液中由水电离出的
c(OH—)= mol·L-1。
(2)第②组情况表明,c 0.2。该混合液中离子浓度c(B+) c(Cl—)(选填“<”、“>”或“=”)。
(3)从第③组实验结果分析,混合溶液中 (选填“<”、“>”或“=”)
甲:BOH的电离程度 BCl的水解程度
乙:c(B+)—2 c (OH—) c(BOH)—2 c(H+)
某学生用0.1000 mol·L-1标准氢氧化钠溶液滴定未知浓度的稀硫酸,其操作可分解为如下几步:
| A.取20.00mL待测硫酸溶液注入洁净的锥形瓶中,并加入2~3滴酚酞试液 |
| B.用标准氢氧化钠溶液润洗滴定管2~3次 |
| C.把盛有标准氢氧化钠溶液碱式滴定管固定好,调节滴定管尖嘴使之充满溶液 |
| D.取标准氢氧化钠溶液注入碱式滴定管至“0”刻度以上2~3mL |
F.把锥形瓶放在滴定管的下面,用标准氢氧化钠溶液滴定至终点并记下滴定管的读数
(1)正确操作步骤的顺序为 (填序号);
(2)上述B步骤操作目的是 ;
(3)判断到达滴定终点的现象是 ;
(4)①达到滴定终点后发现碱式滴定管尖嘴部分有气泡,测定结果会 (填“偏大”“偏小”或“无影响”,下同);
②取待测硫酸溶液的酸式滴定管用蒸馏水洗涤后没有用该硫酸溶液润洗,测定结果会 ;
(5)请在右边的方框内画出正在排气泡的碱式滴定管(仅画出刻度以下部分):

(6)完成3次平行实验,平均消耗标准氢氧化钠溶液体积为20.20mL,则待测液的物质的量浓度为 mol·L-1。
某研究性学习小组为了探究醋酸的电离情况,进行了如下实验。
实验一 配制并标定醋酸溶液的浓度
取冰醋酸配制250 mL 0.2 mol·L-1的醋酸溶液,用0.2 mol·L-1的醋酸溶液稀释成所需浓度的溶液,再用NaOH标准溶液对所配醋酸溶液的浓度进行标定。回答下列问题:
(1)配制250 mL 0.2 mol·L-1醋酸溶液时需要用到的玻璃仪器有量筒、烧杯、玻璃棒、__________________和______________。
(2)为标定某醋酸溶液的准确浓度,用0.200 0 mol·L-1的NaOH溶液对20.00 mL醋酸溶液进行滴定,几次滴定消耗NaOH溶液的体积如下:
| 实验序号 | 1 | 2 | 3 | 4 |
| 消耗NaOH溶液的体积(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
则该醋酸溶液的准确浓度为____________________。(保留小数点后四位)
实验二 探究浓度对醋酸电离程度的影响
用pH计测定25℃时不同浓度的醋酸溶液的pH,结果如下:
| 醋酸溶液浓度(mol·L-1) | 0.001 0 | 0.010 0 | 0.020 0 | 0.100 0 | 0.200 0 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
回答下列问题:
(1)根据表中数据,可以得出醋酸是弱电解质的结论,你认为得出此结论的依据是______________________________________
(2)从表中的数据,还可以得出另一结论:随着醋酸溶液浓度的减小,醋酸的电离程度________(填“增大”“减小”或“不变”)。
实验三 探究温度对醋酸电离程度的影响
请你设计一个实验完成该探究,请简述你的实验方案
________________________________________
 a.c(Na+)= c(H2S)+c(HS?)+2c(S2?)
a.c(Na+)= c(H2S)+c(HS?)+2c(S2?) (4)工业上用硫碘开路循环联产氢气和硫酸的工艺流程如下图所示:
(4)工业上用硫碘开路循环联产氢气和硫酸的工艺流程如下图所示:
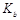 = mol·L-1。
= mol·L-1。