��Ŀ����
��l�������£����ȡ0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ���û��Һ��pH��8�����Һ����ˮ�������OH-Ũ����0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ ��
��2����ͬ�¶��£����������ᱵ����ֱ������ͬ����Ģ�0.1mol��L-1��������Һ
��0.1mol��L-1�Ȼ�����Һ������ˮ��0.1mol��L-1������Һ�У�Ba2+Ũ���ɴ�С��˳���� �����������д��
��3�������£���a mol��L-1�İ�ˮ��0.1mol��L-1������������ϣ�����Һ��c��NH4+����c(Cl-��ʱ���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��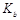 = mol��L-1��
= mol��L-1��
��6�֣�
��1��107��1(2��)
��2����>��>��>�� (2��)
��3��10-8/(a-0.1) (2��)
�������������
��1��0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ��γ�NaA��Һ�����Һ��pH��8��Ϊǿ�������Σ����Һ����ˮ�������OH-Ũ�Ⱦ�����Һ��OH-Ũ��=Kw/c(H+) =10-7 mol��L-1 , 0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ�ȵ�����Һ��H+Ũ��=10-13 mol��L-1����Ϊ107��1 ��
��2������BaSO4��s�� Ba2+��aq��+SO42-��aq���ij����ܽ�ƽ�⣬��0.1mol��L-1�Ȼ�����Һ���д�����Ba2+����0.1mol��L-1��������Һ�͢�0.1mol��L-1������Һ��ʹƽ�������ƶ��� ���Ԣ�>��>��>�� ��
Ba2+��aq��+SO42-��aq���ij����ܽ�ƽ�⣬��0.1mol��L-1�Ȼ�����Һ���д�����Ba2+����0.1mol��L-1��������Һ�͢�0.1mol��L-1������Һ��ʹƽ�������ƶ��� ���Ԣ�>��>��>�� ��
��3����Һ��c��NH4+����c(Cl-��=0.1mol��L-1/2����ϵ���غ��c��H+����c(OH-��=10-7 mol��L-1��NH3��H2O�ĵ��볣��Kb=
���㣺����������Һ�Ļ�ѧ���㡢�����ܽ�ƽ�⡢�����غ���ۺ�Ӧ�õ����֪ʶ��

��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��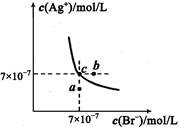
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ����0.10 mol��L��1 NaOH����Һ���вⶨ�����Ũ�ȵ�ʵ�顣ȡ20.00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡������������գ�
| ʵ���� | ������������(mL) | NaOH��Һ��Ũ��(mol��L��1) | �ζ����ʱ��NaOH��Һ��������(mL) |
| 1 | 20.00 | 0.10 | 24.18 |
| 2 | 20.00 | 0.10 | 23.06 |
| 3 | 20.00 | 0.10 | 22.96 |
��1���ζ��ﵽ�յ�ı�־�� ��
��2�������������ݣ��ɼ�����������Ũ��ԼΪ (����С�������λ)��
��3�����ζ�����ʱ����ʽ�ζ����е�Һ����ͼ��ʾ���յ����Ϊ mL��

��4��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��� ��
A������ʽ�ζ���ȡ20.00 mL�������ᣬʹ��ǰ��ˮϴ��δ�ô���������ϴ
B����ƿˮϴ��δ����
C������NaOH����ʱ����С����NaOH����
D���ζ��յ����ʱ����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
���и��仯������ԭ��صķ�Ӧ����
| A���ڿ����н���������Ѹ�������γɱ����� |
| B���Ӻ�ˮ��ͨ����ѧ�����õ�����þ |
| C�����ȵ���˿����ˮ�Ӵ��������������ɫ������ |
| D��п��ϡ���ᷴӦʱ������������CuSO4��Һ��ʹ��Ӧ�ӿ� |
 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�

 2BO3(g)����H=��196.6kJ/mol
2BO3(g)����H=��196.6kJ/mol