��Ŀ����
�����£���ijһԪ��BOH��HCl��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HCl�����ʵ���Ũ�� ��mol��L-1�� | BOH�����ʵ���Ũ�� ��mol��L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=5 |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH>7 |
��ش�
��1���ӵڢ������������BOH�� ��ѡ�ǿ�����������������û����Һ����ˮ�������
c��OH����= mol��L-1��
��2���ڢ������������c 0.2���û��Һ������Ũ��c��B+�� c��Cl����(ѡ�<������>����=��)��
��3���ӵڢ���ʵ���������������Һ�� (ѡ�<������>����=��)
�ף�BOH�ĵ���̶� BCl��ˮ��̶�
�ң�c(B+)��2 c (OH��) c(BOH)��2 c(H+)
��12�֣���ÿ��2�֣�
��1�� ��� 1��10��5
��2��<�� =
��3�� >�� =
���������������1�������ʵ�����һԪ�ᡢ����ʱ��Һ�����ԣ�������������Һ�������Ӿ�����������ˮ��õ��ӵģ�������Һ��ˮ�������c��OH����=c��H����=1��10-5mol��L��1���ʴ�Ϊ�����1��10-5��
��2��BOHΪ����������Ũ�Ȼ����Һ��pHС��7����Ϊ��֤pH=7��Ӧʹ��Ũ��С��0.2mol��L��1����Һ�����ԣ���Һ��c��OH����=c��H��������Һ�ʵ����ԣ����ڵ���غ㣬����c��B����=c��Cl�������ʴ�Ϊ������=��
��3���ɢ���ʵ������֪����Ϻ�ΪBOH��BCl�Ļ��Һ��pH��7����ĵ�������ε�ˮ�⣬��Һ�д��ڵ���غ�c��H����+c��B�� ��=c��OH����+c��Cl�� �������������غ��c��B����+c��BOH��=2c��Cl�� ������������ʽ������c��B����-2c ��OH����=c��BOH��-2c��H�������ʴ�Ϊ������=��
���㣺�����ʱ�Ķ����жϼ��й�pH�ļ���

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д���֪��������ʱH2O H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH  H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH���ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ�������� �������� ���������¶�ʱ��C(OH��)�������������������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵΪ�������� ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������ʱ������pH��3��HA��ҺV1mL��pH��11��NaOH��ҺV2 mL��ϣ�������˵������ȷ����____________��
| A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1 |
| B����V1=V2����Ӧ����ҺpHһ������7 |
| C������Ӧ����Һ�����ԣ���V1һ������V2 |
| D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2 |
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ��������� �����ɳ�����ԭ�������� ������������������������������������������������������ ����
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��
KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ��mol/L��
�ҹ�ij���͵��ͭ������ҵ����ұ��������ͭ��������ʴﵽ97����87������ͼ��ʾ��ұ���ӹ������̣�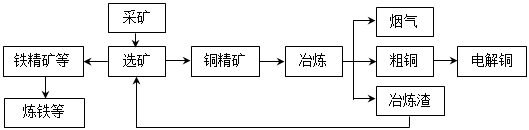
ұ���е���Ҫ��Ӧ��Cu2S + O2 =" 2Cu" + SO2
��1�������е���Ҫ������________________���������Դ�����ʺͼ��ſ��ǣ����ۺ����÷�ʽ����___________��
��2����ⷨ��ͭʱ��������____________�����ͭ�塱��ͭ�塱������ͭ�к��еĽ����Ե��ʵ���ʽ�ڵ���_______________������������������IJ۵ף������ĵ缫��Ӧʽ��_________________________________________��
��3���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣
�������ʵ��ܶȻ�������KSP����
| ���� | Fe(OH)2 | Fe(OH)3 | Zn(OH)2 | Cu(OH)2 |
| KSP | 8.0��10��16 | 4.0��10��38 | 3.0��10��17 | 2.2��10��20 |
���ڵ��Һ��pH�dz�ȥ�������ӵij��÷����������ϱ����ܶȻ������жϣ����е����ʵ���Ũ�ȵ�Fe2+��Zn2+��Fe3+��Cu2+����Һ����pH�������ȳ���������������______________��
һ�ַ������ȼ��������H2O2���ٵ���pH��4���ҡ�����H2O2������Ӧ�����ӷ���ʽΪ___________________________________________________________________________��
��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��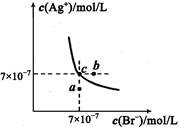
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�

 �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
 SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-�� 2BO3(g)����H=��196.6kJ/mol
2BO3(g)����H=��196.6kJ/mol