��Ŀ����
17��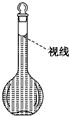 ʵ������Ҫ����0.50mol/L NaCl��Һ500mL�������в������������ʵ������֣���ʹ��������������
ʵ������Ҫ����0.50mol/L NaCl��Һ500mL�������в������������ʵ������֣���ʹ����������������1��ѡ����������ɱ�ʵ��������������У�������ƽ����ȷ��0.1g����ҩ�ס��ձ�����������500 mL����ƿ����ͷ�ι��Լ�����������Ƭ��ֽ��
��2�����㣮���Ƹ���Һ��ȡNaCl����14.6g��
��3��������
����ƽ��ƽ��
�ڳ�����ϣ���ҩƷ�����ձ��У�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ���ǽ��裬����NaCl�ܽ⣮
��5��ת�ơ�ϴ�ӣ���ת��ʱӦʹ�ò�������Ŀ��������������Ҫϴ���ձ���������2��3����Ϊ�˱�֤����ȫ��ת��������ƿ��
��6�����ݣ�ҡ�ȣ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȣ�
��8�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�ƫ�ͣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2������n=cV��m=nM�����㣻
��4��ͨ���������Ľ�����Լ����ܽ⣻
��5��Ϊ�˷�ֹ��Һ������Ҫ�ò�����������ϴ���ձ�����ϴ��Һע������ƿ��Ŀ���ǽ����ʶ�ת��������ƿ��
��8������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮������480ml������ƿ����ѡ��500ml����ƿ����ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������500ml����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��2��������480ml������ƿ����ѡ��500ml����ƿ�������Ƴ�����Һ�����Ϊ500ml������n=cV��֪��Ҫ��NaCl�����ʵ���n=0.50mol/L��0.5L=0.25mol������m=nM=0.25mol��58.5g/mol=14.6g���ʴ�Ϊ��14.6��
��4���ܽ�ʱ����Ҫʹ�ò��������裬�����Ȼ��Ƶ��ܽ⣬�ʴ�Ϊ�����裬�����ܽ⣻
��5��Ϊ�˷�ֹ��Һ������Ҫ�ò�����������ϴ���ձ�����ϴ��Һע������ƿ��Ŀ���ǽ����ʶ�ת��������ƿ���ʴ�Ϊ����������֤����ȫ��ת��������ƿ�У�
��8�����ӿ̶��ߣ��ᵼ����Һ���ƫ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| A�� | 17.87kJ | B�� | -17.87kJ/mol | C�� | 411 kJ/mol | D�� | -411 kJ/mol |
| A�� | K+��Na+��Cl-��SO42- | B�� | NH4+��Na+��Cl-��HCO3- | ||
| C�� | K+��Na+��NO3-��SO42- | D�� | K+��Na+��OH-��SO42- |
| A�� | ��ԭ����KClO3 | B�� | HCl����ԭ | ||
| C�� | �õ�3molCl2ת��6mol���� | D�� | ���������ԭ���ﶼ��Cl2 |
| A�� | ������������Ӧ | B�� | �������������ᷴӦ | ||
| C�� | �����뻯������û���Ӧ | D�� | �������������� |
| A�� | ʳ��ˮ | B�� | ��ˮ | C�� | Fe��OH��3���� | D�� | ����ͭ��Һ |
| A�� | ����ʪ��pH��ֽ�ⶨ0.1mol/L��ˮ��pHֵ | |
| B�� | ��CaCO3������Һ�У�����Na2CO3���壬ƽ��ʱc��Ca2+��=c��CO${\;}_{3}^{2-}$��=$\sqrt{{K}_{sp}CaC{O}_{3}}$ | |
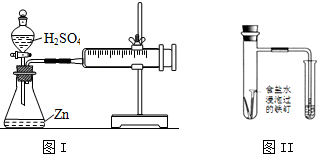
| C�� | ͼIװ�ÿ�����̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� | |
| D�� | �۲�ͼII������ˮ���ı仯����֪��������������Ҫ���������ⸯʴ |
N2��g��+2O2��g��=N2O4��g����H=+8.7kJ•mol-1
N2H4��g��+O2��g��=N2��g��+2H2O��g����H=-534.0kJ•mol-1
���б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ���ǣ�������
| A�� | 2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-542.7 kJ•mol-1 | |
| B�� | 2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-1059.3 kJ•mol-1 | |
| C�� | N2H4��g��+$\frac{1}{2}$N2O4��g��=$\frac{3}{2}$N2��g��+2H2O��g����H=-1076.7 kJ•mol-1 | |
| D�� | 2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-1076.7 kJ•mol-1 |