��Ŀ����
��������(ClO2)��һ�ֹ��ס���Ч����������������ˮ��������ˮ�����ȷ����й㷺Ӧ�á�������ijУ��ѧ�о�С�����ʵ������ȡClO2�Ĺ�������ͼ�������й�˵����ȷ���ǣ� ��


A�����ʱ������Ӧ�Ļ�ѧ����ʽΪ2HCl Cl2����H2�� Cl2����H2�� |
| B����NaClO2��Һ��ȡ0.6 mol ClO2ʱ����������0.1 mol NCl3 |
| C���������Ļ������ͨ��ʢ�м�ʯ�ҵĸ������Գ�ȥClO2�е�NH3 |
| D����ҺX����Ҫ�ɷ�ΪNaClO2��NaOH |
B
��������ͼ��֪�����ʱ�ķ�Ӧ��ΪNH4Cl��HCl������ΪH2��NCl3����ȷ�Ļ�ѧ����ʽΪNH4Cl��2HCl NCl3��3H2����A������ʯ������ȥNH3��C��������������ԭ��Ӧ���ɺ�Ԫ���غ��֪����ҺX����Ҫ�ɷ�ΪNaCl��NaOH��
NCl3��3H2����A������ʯ������ȥNH3��C��������������ԭ��Ӧ���ɺ�Ԫ���غ��֪����ҺX����Ҫ�ɷ�ΪNaCl��NaOH��
 NCl3��3H2����A������ʯ������ȥNH3��C��������������ԭ��Ӧ���ɺ�Ԫ���غ��֪����ҺX����Ҫ�ɷ�ΪNaCl��NaOH��
NCl3��3H2����A������ʯ������ȥNH3��C��������������ԭ��Ӧ���ɺ�Ԫ���غ��֪����ҺX����Ҫ�ɷ�ΪNaCl��NaOH��
��ϰ��ϵ�д�
 ��������������������ϵ�д�
��������������������ϵ�д�
�����Ŀ

 ��
�� 3Cu2++2R+yH2O��
3Cu2++2R+yH2O��
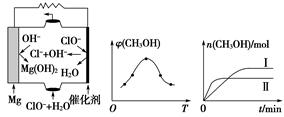
 CH3OH��g����H2O��g������H��
CH3OH��g����H2O��g������H��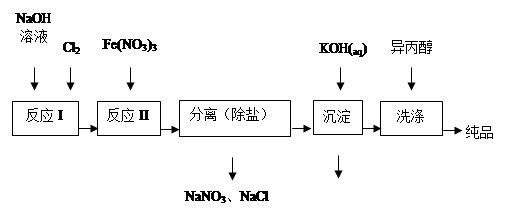 ��ϴ�Ӵ�Ʒʱѡ�������������ˮ�������ǣ� ��
��ϴ�Ӵ�Ʒʱѡ�������������ˮ�������ǣ� ��