��Ŀ����
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�

(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��

(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��
(1)SiO2��2KOH=K2SiO3��H2O
(2)6KOH��KClO3��3MnO2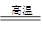 KCl��3K2MnO4��3H2O
KCl��3K2MnO4��3H2O
(3)66.7 %
(4)���ٹ���ʱ�����(���ֹ���¹�����KMnO4���������)
(5)���ˡ��¶ȹ���KMnO4��ֽ�
(2)6KOH��KClO3��3MnO2
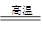 KCl��3K2MnO4��3H2O
KCl��3K2MnO4��3H2O(3)66.7 %
(4)���ٹ���ʱ�����(���ֹ���¹�����KMnO4���������)
(5)���ˡ��¶ȹ���KMnO4��ֽ�
�������Ƚ�MnO2ת��ΪK2MnO4��K2MnO4��������������ԭ��Ӧ����KMnO4��(1)�ڢٲ��в��������������ô����������ں��ߺ��е�SiO2�ڸ����»���KOH������Ӧ��SiO2��2KOH=K2SiO3��H2O��(2)KOH��KClO3��MnO2����ʱ��MnO2������ΪK2MnO4��KClO3����ԭΪKCl����ѧ����ʽΪ6KOH��KClO3��3MnO2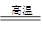 KCl��3K2MnO4��3H2O��(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ�����ݵ�ʧ�����غ��֪����MnO4����MnO2�����ʵ���֮��Ϊ2��1����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ66.7 %��(4)�ڢݲ����ȹ��˵�Ŀ���Ƿ�ֹ���¹�����KMnO4��������ġ�(5)��������У��¶Ȳ��˹��ߣ�����KMnO4��ֽ⡣
KCl��3K2MnO4��3H2O��(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ�����ݵ�ʧ�����غ��֪����MnO4����MnO2�����ʵ���֮��Ϊ2��1����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ66.7 %��(4)�ڢݲ����ȹ��˵�Ŀ���Ƿ�ֹ���¹�����KMnO4��������ġ�(5)��������У��¶Ȳ��˹��ߣ�����KMnO4��ֽ⡣
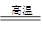 KCl��3K2MnO4��3H2O��(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ�����ݵ�ʧ�����غ��֪����MnO4����MnO2�����ʵ���֮��Ϊ2��1����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ66.7 %��(4)�ڢݲ����ȹ��˵�Ŀ���Ƿ�ֹ���¹�����KMnO4��������ġ�(5)��������У��¶Ȳ��˹��ߣ�����KMnO4��ֽ⡣
KCl��3K2MnO4��3H2O��(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ�����ݵ�ʧ�����غ��֪����MnO4����MnO2�����ʵ���֮��Ϊ2��1����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ66.7 %��(4)�ڢݲ����ȹ��˵�Ŀ���Ƿ�ֹ���¹�����KMnO4��������ġ�(5)��������У��¶Ȳ��˹��ߣ�����KMnO4��ֽ⡣
��ϰ��ϵ�д�
�����Ŀ

 Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
 Cl2����H2��
Cl2����H2�� Fe(OH)3��5OH��]
Fe(OH)3��5OH��]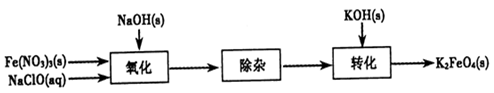
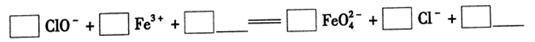
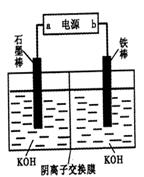
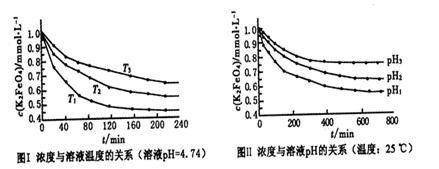

 ��2FeS��SO2������ƽ���������й��ڸ÷�Ӧ��˵��������ǣ���������
��2FeS��SO2������ƽ���������й��ڸ÷�Ӧ��˵��������ǣ���������