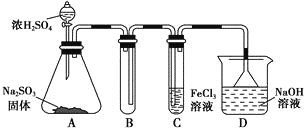
��Ŀ����
����Ŀ���Ӻ�ˮ�п�����ȡ�ܶ����õ����ʣ�����Ӻ�ˮ�������õ���±ˮ�п�����ȡ�⡣����̿�������ǹ�ҵ��ȡ��ķ���֮һ�����������£�

������ʾ��
��.pH=2 ʱ�� NaNO2 ��Һֻ�ܽ� I������Ϊ I2��ͬʱ���� NO��
��.I2+5Cl2+6H2O=2HIO3+10HCl��
��.I2�ڼ�����Һ�з�Ӧ����I����IO3����
(1)��Ӧ�ٵ����ӷ���ʽ_____��
(2)�������У����� I2�����ԣ�������� X ��������_____��
(3)Cl2������ KMnO4 �ȶ��dz��õ�ǿ�����������ù���������±ˮ�е� I��ȴѡ���˼۸�ϸߵ�NaNO2��ԭ����_____��
(4)�������У���֪��Ӧ�۹��˺���Һ���Դ��������� I2��I����IO3���� �������Һ�е�I������ʵ�鷽������������ʵ���пɹ�ѡ����Լ���ϡ H2SO4��������Һ��Fe2(SO4)3 ��Һ��
A.��Һ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڡ�
B._____��
���𰸡�2NO2��+2 I��+4H+ =I2+2NO+2H2O ����(�����)�������ᾧ ���������Ը�����صȶ��dz��õ�ǿ�����������������I2 ��ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I��
��������
(1)����Ϣ֪����������Һ�У�NaNO2��Һ��I������Ϊ I2��ͬʱ���� NO��
(2)����̿������õ���ͻ���̿�Ĺ�����������õ����������������ʣ����ü��ȵķ���������ת��Ϊ������Ȼ������ȴ�ᾧ��ù���⣻
(3)Cl2������ KMnO4���dz��õ�ǿ���������ܽ�I-����ΪI2���ٽ�I2��������Ϊ����̬�ĺ������ӣ���NaNO2��I-��Ӧ��ֻ������I2��
(4)Ҫ����I-�Ĵ��ڣ��轫I-����ΪI2�������õ��ۼ���I2�Ĵ��ڡ�����ԭ�е�I2�����I-����I2�ļ��飬����������I-ǰ���轫I2��ȥ��
(1)��Ӧ���У���������Һ�У�NaNO2��Һ��I������Ϊ I2��ͬʱ���� NO�����ӷ���ʽΪ2NO2��+2 I��+4H+ =I2+2NO+2H2O����Ϊ��2NO2��+2 I��+4H+ =I2+2NO+2H2O��
(2)����̿������õ���ͻ���̿�Ĺ�������������У������õ����������������ʡ��ɴ˵ó�������� X ������������(�����)�������ᾧ����Ϊ������(�����)�������ᾧ��
(3)��ʹ�ü۸�ϵ͵�Cl2������ KMnO4 �ȳ��õ�ǿ����������Ȼ�ǶԲ��ﲻ����Ҳ���Dz�������ͣ��������I2��һ������������������ɴ˵ó��˸ù���������±ˮ�е� I��ȴѡ���˼۸�ϸߵ�NaNO2��ԭ�������������Ը�����صȶ��dz��õ�ǿ�����������������I2����Ϊ�����������Ը�����صȶ��dz��õ�ǿ�����������������I2��
(4) �������У���֪��Ӧ�۹��˺���Һ���Դ��������� I2��I����IO3����Ҫ����I-�Ĵ��ڣ��轫I-����ΪI2�������õ��ۼ���I2�Ĵ��ڡ�����ԭ�е�I2�����I-����I2�ļ��飬����������I-ǰ���轫I2��ȥ��ʵ�鷽��Ϊ��
A.��Һ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڡ�
B. ��ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I������Ϊ����ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I����

����Ŀ��ʵ������NaHSO4��Ba(OH)2��NH3��H2O��NaHCO3��KAl(SO4)2������ɫ��Һ������ͨ������֮������Ӧ�����������м��𡣲������ʼ�ķ�Ӧ���������
A | B | C | D | E | |
A | �� | ||||
B | �� | �� | |||
C | �� | ���� | �� | ||
D | �� | ���� | �� | ||
E | �� | �� | �� |
������������ʾ�����������ʣ���������ʾ���ɳ���������������Ϣ���ش��������⡣
(1)B��E�Ļ�ѧʽ�ֱ�Ϊ________��________��
(2)д��A�ĵ��뷽��ʽ��_____________��