��Ŀ����
(12��)����ѧ�������ʽṹ�����ʡ�
ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ������ͭ������ɱ�����ȡ�
��1��Cu2+�ĺ�������Ų�ʽΪ________________��
��2����ѧ��ͨ��X���߲���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�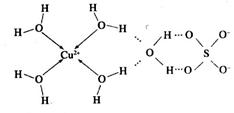
�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ______��
�ڵ�����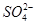 �Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
�Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
��3��������ͭ��Һ�м��������ˮ�������� �����ӡ���֪
�����ӡ���֪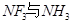 �Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
��4�� N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��
N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��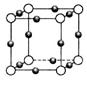
��1��[Ar]3d9
��2����[Cu(H2O)4]SO4��H2O ������������ sp3
��3��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ�������
��4��6 206/a3b
���������������1��ͭΪ29��Ԫ�أ����̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d104s1������3d��4s����ֱ�Ϊȫ�����Ͱ��������Cu2+�ĺ�������Ų�Ϊ[Ar]3d9����2�����ݵ����Ľṹʾ��ͼ��֪Cu2+��4��H2O�е���ԭ���γ���λ���������е�2��ˮ������������һ��H2O�������ϣ�Ȼ������������������ϣ��ʿɱ�ʾΪ��[Cu(H2O)4]SO4��H2O������ͼʾ��֪������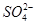 �Ŀռ乹��ӦΪ���������ͣ�H2O��Oԭ�ӵ��ӻ���ʽΪsp3����3����ΪF�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ������ӣ���4�����ݾ����ṹ��֪����λ��ΪN3-������ΪCu+����N3-����λ����6��1�������к���3��Cu+��1��N3������1������������Ϊ206/NA g�����Ϊa3 cm3�������ܶ�bg/cm3=206/NA g��a3 cm3����NA=206/a3b��
�Ŀռ乹��ӦΪ���������ͣ�H2O��Oԭ�ӵ��ӻ���ʽΪsp3����3����ΪF�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ������ӣ���4�����ݾ����ṹ��֪����λ��ΪN3-������ΪCu+����N3-����λ����6��1�������к���3��Cu+��1��N3������1������������Ϊ206/NA g�����Ϊa3 cm3�������ܶ�bg/cm3=206/NA g��a3 cm3����NA=206/a3b��
���㣺����ԭ�Ӻ�������Ų����ɡ���λ�������ӹ��͡�����֪ʶ�����ݡ�

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�[��ѧ��ѡ��3���ʽṹ������](15��)
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ�����ڱ���_________������ļ۵����Ų�ʽΪ____________________��
��2���ڲ�̫ϡ����Һ�У���������Զ����ӵ�(HF)2��ʽ���ڵġ�ʹ�������ӵϵ���������________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________��
| | �� | �� | �� | �� | �� |
| ��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4����������� ������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4����������� ��������������5������ˮ�е��ܽ����ȻС�����ڵ⻯����Һ���ܽ��ȴ������������������Һ�з������з�ӦI-+I2=I3-��I3-���ӵ�����ԭ����Χ�Ҽ����ӶԶ���Ϊ_____���µ��ӶԶ���Ϊ______�� I3-���ӵĿռ乹��Ϊ___________��
��KI3���Ƶģ�����CsICl2������֪CsICl2���ȶ��������ֽ⣬���������ɾ����ܸ�������ʣ�����������_____ʽ������ A��CsICl2=CsCl+ICl B��CsICl2=CsI+Cl2
��6����֪ClO2-Ϊ���ͣ�������ԭ����Χ���ĶԼ۲���ӡ�ClO2-������ԭ�ӵ��ӻ��������Ϊ___________��д��һ��ClO2-�ĵȵ�����__________��
��7����ͼΪ�⾧�徧���ṹ���й�˵������ȷ����_____________��

A������ӵ�������2�ֲ�ͬ��ȡ��2��ȡ��ͬ
�ĵ������4��λ��������λ�γɲ�ṹ
B���þ�̯����֪ƽ��ÿ����������4����ԭ��
C���⾧��Ϊ��������Ŀռ�ṹ����ԭ�Ӿ���
D���⾧���еĵ�ԭ�Ӽ���ڷǼ��Լ��ͷ��»���
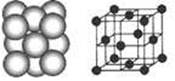
��8����֪CaF2���壨����ͼ�����ܶ�Ϊ��g/cm3��NAΪ�����ӵ����������ڵ�����Ca2+�ĺ˼��Ϊa cm����CaF2����Է����������Ա�ʾΪ___________��

��15�֣���������Ԫ�ص����ʻ�ṹ��Ϣ���£�
| Ԫ�ر�� | �����Ϣ |
| X | �ؿ��к������Ľ���Ԫ�أ�Ԫ�����Ϊ+3�ۡ� |
| Y | ԭ�������������ǵ��Ӳ�����2����������������X������������2���� |
| Z | ͬ��������Ԫ����ԭ�Ӱ뾶��С�������µ��ʳ�Һ̬�� |
| M | �ܴӺ�ˮ����ȡ�Ľ���Ԫ�أ����ʿ��ڵ����������̼��ȼ�ա� |
��2��Y����������Ũ���������������Ļ�ѧʽΪ ��
��3��������Z���ʱ�Y���ʻ�����ǿ��һ����ѧ����ʽ�� ��
��4�������£�������X�ĵ��ʳ���������Ӧ����(��ѡ�����) ��
A��CuSO4��Һ B��Fe2O3 C��Ũ���� D��NaOH��Һ E��Na2CO3����
��5����Ԫ����ZԪ���γɻ�����FeZ3��FeZ3��Һ����ͼ��ʾ�������顣װ��ͨ������ӵ�Դ �����������������̼���ߵ�ú����ɫ�������һ̼��������Һ�����ֵ������� ��

A~F��ΪԪ�����ڱ���ǰ������Ԫ�أ��������Ϣ���±���
| Ԫ�� | �� �� �� Ϣ |
| A | A�Ļ�̬ԭ�����������Ų�ʽΪ2s22p3 |
| B | B�ǵؿ��к�����ߵ�Ԫ�� |
| C | C����B�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| D | D��һ�ֺ��ص�������Ϊ64��������Ϊ35 |
| E ��F | E ��F��ͬ������ͬ�壬��ԭ������F��E��2 |
��ش��������⣺
(1)D�ļ۵��ӵĵ����Ų�ʽ�� ��Fԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
(2)A��B�ĵ�һ�����ܵĴ�С˳��Ϊ ��
(3)AB3����Aԭ�ӵ��ӻ��������Ϊ_____����A2B��Ϊ�ȵ�����ķ��ӵķ���ʽΪ (��дһ������)��
(4) D����ľ�����ͼ��ʾΪ�����������ܶѻ�(�ھ����Ķ�������ľ�����һ��Dԭ��)����D�ľ�����Dԭ�ӵ���λ��Ϊ ��
(5)��֪17gA�ļ��⻯�������������̬ˮʱ�ų�QkJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ ��
(6)C2B2�ĵ���ʽΪ____��������E�Ķ��Ȼ�����Һ��Ӧ������Ӧ��C2B2��E�Ķ��Ȼ�������ʵ���֮��Ϊ1��2����÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
 [Cu(NH3)3CO]Ac
[Cu(NH3)3CO]Ac