��Ŀ����
(17��)I����ҵ����һ����CO2�������״�ȼ�ϵķ�����
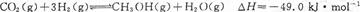
��6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ(ʵ��)��ͼ������a(1��6)��ʾ����1 minʱH2�����ʵ�����6 mol��
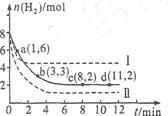
��1��a������Ӧ���� (����ڡ������ڡ���С�ڡ�)�淴Ӧ���ʡ��仯ѧƽ�ⳣ��K=
(2)����ʱ���ƽ����Ӧ���������� ��
(3)���ı�ijһʵ�������ٽ�������ʵ����H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı��� �����ߢ��Ӧ��ʵ�������ı��� ����������ٳ���3molCO2��4 mol H2,H2O(g)��������� ����������䡱��С����
��ijʵ��С����̽����Ӧ�������¶ȵĹ�ϵ,����1mol��L��KI��Һ��0��1mol��L��H2S04��Һ��������Һ����ʵ��ʱ�⼸���Լ��ļ���˳��Ϊ��KI��Һ�� �� ��
��Ӧ�ķ���ʽΪ
��. ������Ʒ�к�Fe��Zn��Ag��Cu�����ֽ������ʣ�Ϊ��øߴ��ȵ�����ij��ȤС��ͬѧ����Ǧ����Ϊ��Դ��������ʯīΪ�缫�������������Һ�Դ��������ᴿ��
(1)�������������������ij������У���Ҫ�Ľ�������Ϊ (�ѧʽ)��
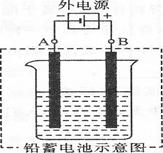
(2) ������ͼ��ʾ���Ӷ�Ǧ���ؽ��г�硣���һ��ʱ�������A�缫������ (�ѧʽ)��B�缫�ϵĵ缫��ӦʽΪ �������ϡ�Ǧ���ص������� ��(�A����B��)��
(3)���ü���ȼ�ϵ��Ϊ��Դ����25�桢101 kPaʱ����CH4��������ֱ��ȼ������1 molˮ��������401 kJ����l gˮ����ת����Һ̬ˮ����2��445 kJ����CH4��ȼ����Ϊ (ȡ����)kJ��mol-��
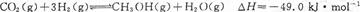
��6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ(ʵ��)��ͼ������a(1��6)��ʾ����1 minʱH2�����ʵ�����6 mol��
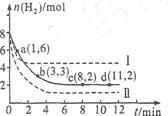
��1��a������Ӧ���� (����ڡ������ڡ���С�ڡ�)�淴Ӧ���ʡ��仯ѧƽ�ⳣ��K=
(2)����ʱ���ƽ����Ӧ���������� ��
| A��O��1 min | B��1��3 min | C��3��8 min | D��8��11 min |
��ijʵ��С����̽����Ӧ�������¶ȵĹ�ϵ,����1mol��L��KI��Һ��0��1mol��L��H2S04��Һ��������Һ����ʵ��ʱ�⼸���Լ��ļ���˳��Ϊ��KI��Һ�� �� ��
��Ӧ�ķ���ʽΪ
��. ������Ʒ�к�Fe��Zn��Ag��Cu�����ֽ������ʣ�Ϊ��øߴ��ȵ�����ij��ȤС��ͬѧ����Ǧ����Ϊ��Դ��������ʯīΪ�缫�������������Һ�Դ��������ᴿ��
(1)�������������������ij������У���Ҫ�Ľ�������Ϊ (�ѧʽ)��

(2) ������ͼ��ʾ���Ӷ�Ǧ���ؽ��г�硣���һ��ʱ�������A�缫������ (�ѧʽ)��B�缫�ϵĵ缫��ӦʽΪ �������ϡ�Ǧ���ص������� ��(�A����B��)��
(3)���ü���ȼ�ϵ��Ϊ��Դ����25�桢101 kPaʱ����CH4��������ֱ��ȼ������1 molˮ��������401 kJ����l gˮ����ת����Һ̬ˮ����2��445 kJ����CH4��ȼ����Ϊ (ȡ����)kJ��mol-��
��(1)���� 1/2 (2) A (3) �����¶� ����ѹǿ ���� �������Һ��H2S04��Һ 4H��4I����O2=2I2��2H2O
�� (1)Ag��Cu (2)Pb PbSO4��2e����2H2O=PbO2��SO42����4H�� B (3) 890
�� (1)Ag��Cu (2)Pb PbSO4��2e����2H2O=PbO2��SO42����4H�� B (3) 890
��1��a�����������ʵ��������ڼ�С�ģ����Է�Ӧ����������Ӧ������У��������Ӧ���ʴ����淴Ӧ���ʡ�ƽ��ʱ������2mol��������������6mol������ͬʱ����CO2��2mol�����ɼ״���ˮ������2mol������ƽ�ⳣ������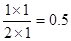 ��
��
��2����Ӧ����Խ��ӳ��������б�ʾ�Խ�����Է�Ӧ����������O��1 min����A����ȷ�ġ�
��3������ͼ���֪����I��Ӧ�ķ�Ӧ���ʿ죬ƽ��ʱ���������ʵ�����˵��ƽ�������淴Ӧ�����ƶ��ģ���˸ı�������������¶ȡ����ߢ�ķ�Ӧ����Ҳ�죬��ƽ��ʱ���������ʵ���С��˵��������Ӧ�����ƶ������������ѹǿ����������ٳ���3molCO2��4 mol H2,�൱������ѹǿ��ƽ��������Ӧ�����ƶ�������ˮ�����ĺ�������
�����ڵ������ױ�������������������ɫ�����Լ����˳���ǵ�����Һ��H2S04��Һ������ʽΪ4H��4I����O2=2I2��2H2O��
��.��1������ͭ�Ľ������������ģ����������е���Ҫ������ͭ������
��2��A�͵�Դ�ĸ������������������缫������Ǧ��B�͵�Դ���������������������缫��ӦʽΪPbSO4��2e����2H2O=PbO2��SO42����4H����B������������Ǧ������B��������
��3��l gˮ����ת����Һ̬ˮ����2��445 kJ������1molˮ����ת����Һ̬ˮ������2.445kJ��18��44.01kJ������1molˮ�����ų���������401kJ����������1molҺ̬ˮ�ų���������401kJ��44.01kJ��445.01kJ�����Լ����ȼ������445.01kJ��2mol��890kJ/mol��
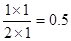 ��
����2����Ӧ����Խ��ӳ��������б�ʾ�Խ�����Է�Ӧ����������O��1 min����A����ȷ�ġ�
��3������ͼ���֪����I��Ӧ�ķ�Ӧ���ʿ죬ƽ��ʱ���������ʵ�����˵��ƽ�������淴Ӧ�����ƶ��ģ���˸ı�������������¶ȡ����ߢ�ķ�Ӧ����Ҳ�죬��ƽ��ʱ���������ʵ���С��˵��������Ӧ�����ƶ������������ѹǿ����������ٳ���3molCO2��4 mol H2,�൱������ѹǿ��ƽ��������Ӧ�����ƶ�������ˮ�����ĺ�������
�����ڵ������ױ�������������������ɫ�����Լ����˳���ǵ�����Һ��H2S04��Һ������ʽΪ4H��4I����O2=2I2��2H2O��
��.��1������ͭ�Ľ������������ģ����������е���Ҫ������ͭ������
��2��A�͵�Դ�ĸ������������������缫������Ǧ��B�͵�Դ���������������������缫��ӦʽΪPbSO4��2e����2H2O=PbO2��SO42����4H����B������������Ǧ������B��������
��3��l gˮ����ת����Һ̬ˮ����2��445 kJ������1molˮ����ת����Һ̬ˮ������2.445kJ��18��44.01kJ������1molˮ�����ų���������401kJ����������1molҺ̬ˮ�ų���������401kJ��44.01kJ��445.01kJ�����Լ����ȼ������445.01kJ��2mol��890kJ/mol��

��ϰ��ϵ�д�
�����Ŀ
 H2NCOONH4(���������)(l) ��H1
H2NCOONH4(���������)(l) ��H1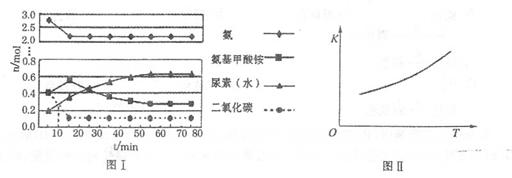
 ��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
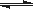 Si3N4��s��+6CO��g��
Si3N4��s��+6CO��g��
 H2(g)+CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���Իش��������⣺
H2(g)+CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���Իش��������⣺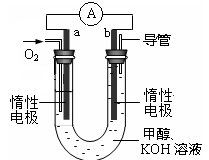
 ����ȷ����
����ȷ����

 C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����
C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����