��Ŀ����
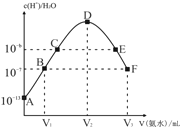
����Ŀ����֪������,Ka1(H2CO3)=4.3��10-7,Ka2(H2CO3)=5.6��10-11��ij��Ԫ��H2R�������ε���Һ��,H2R��HR-��R2-���ߵ����ʵ�����������ҺpH�仯��ϵ��ͼ��ʾ����������������ǣ� ��

A. ��pH=4.3����Һ�У�3c(R2-)=c(Na+)+c(H+)-c(OH-)
B. ���������Ũ�ȵ�NaOH��Һ��H2R��Һ��Ϻ���Һ��ˮ�ĵ���̶ȱȴ�ˮС
C. ��Ũ�ȵ�NaOH��Һ��H2R��Һ�������1��2���,��Һ��pH=1.3
D. ��Na2CO3��Һ�м�������H2R��Һ��������Ӧ��2CO32-+H2R=2HCO3-+R2-
���𰸡�C
��������A.��pH=4.3����Һ�У�c(R2)=c(HR)��2c(R2)+c(HR)+c(OH)=c(Na+)+c(H+)������3c(R2-)=c(Na+)+c(H+)-c(OH-)����A��ȷ��B. �������Ũ�ȵ�NaOH��Һ��H2R��Һ��Ϻ����ɵ�Ũ�ȵ�H2R��HR����Һ��pH=1.3����Һ�����ԣ���ˮ�ĵ������������ã�������Һ��ˮ�ĵ���̶ȱȴ�ˮС����B��ȷ��C.��ͼ��֪����Ũ�ȵ�NaOH��Һ��H2R��Һ�������1��1��Ϻ�,��Һ��pH=1.3����C����D.��pH=1.3ʱ��c(HR)=c(H2R)����H2R��K1=![]() =101.3mol/L����pH=4.4ʱ��c(HR)=c(R2)����H2R��K2=
=101.3mol/L����pH=4.4ʱ��c(HR)=c(R2)����H2R��K2=![]() =104.4mol/L����H2CO3��Ka1=4.2��107,Ka2=5.6��1011���ʿ�֪����ǿ����ϵ��H2R>HR>H2CO3>HCO3������Na2CO3��Һ�м�������H2R��Һ������R2�������ӷ���ʽΪ2CO32-+H2R=2HCO3-+R2-����D��ȷ����ѡC.
=104.4mol/L����H2CO3��Ka1=4.2��107,Ka2=5.6��1011���ʿ�֪����ǿ����ϵ��H2R>HR>H2CO3>HCO3������Na2CO3��Һ�м�������H2R��Һ������R2�������ӷ���ʽΪ2CO32-+H2R=2HCO3-+R2-����D��ȷ����ѡC.

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ����������(POCl3)�������뵼����Ӽ������άԭ��,ʵ�����Ʊ�POCl3���ⶨ��Ʒ������ʵ��������£�
���Ʊ�POCl3��
������������Һ̬PCl3����ȡPOCl3,ʵ��װ��(���ȼ��г�������)��ͼ��ʾ��
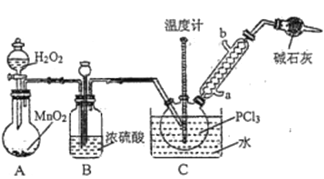
��֪����Ag++SCN-=AgSCN��;Ksp(AgCl)>Ksp(AgSCN);�������������ᡣ
��PCl3��POCl3�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | ������,��Ϊ��ɫҺ��,��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POCl3 | 2.0 | 106.0 | 153.5 |
(1)POCl3��ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
(2)װ��B�����ó�����O2��,����_________________________________________������ܵ�������_______________��
(3)��Ӧ�¶�Ҫ������60~65��,ԭ����_____________________________________________��
�ⶨPOCl3��Ʒ�ĺ�����
ʵ�鲽�裺
���Ʊ�POCl3ʵ�������������ƿ�е�Һ����ȴ�����£�ȷ��ȡ30.7gPOCl3��Ʒ,����ʢ��60.00mL����ˮ��ˮ��ƿ��ҡ������ȫˮ��,��ˮ��Һ���100.00mL��Һ��
��ȡ10.00mL��Һ����ƿ��,����10.00mL3.2mol/LAgNO3����Һ��
�ۼ�����������������ҡ����ʹ�������汻�л��︲�ǡ�
����XΪָʾ��,��0.2mol/LKSCN��Һ�춨������AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥ10.00mLKSCN��Һ��
(4)������м�����������������___________________________________________�����˲���,�����Ʒ����Ԫ�ص�������������__________(�ƫ����ƫС�����䡱)��
(5)�������XΪ__________,��Ʒ��POCl3����������Ϊ____________��