��Ŀ����
4�� ����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú������Ŀǰ���ڿ�����ֱ��������
����Ȼ��Ϊԭ�Ϻϳɼ״������ķ�����ˮú������Ŀǰ���ڿ�����ֱ����������1���й��Ȼ�ѧ����ʽ���£�
ˮú������
CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ��mol-1
CO��g��+2H2?CH3OH��g����H2=-90.1kJ��mol-1
ֱ����������
2CH4��g��+O2��g��?2CH3OH��g����H3=-2511kJ��mol-1
��2����ҵ����������̼������Ҳ�ɺϳɼ״���CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H�����ܱ�������Ͷ��1mol CO2��2.75 mol H2�ڲ�ͬ�����·�����Ӧ��ʵ����ƽƽ��ʱ�״������ʵ������¶ȡ�ѹǿ�ı仯��ͼ��ʾ
�ٶ�����̼�ϳɼ״�����Ӧ�ġ�H��0���������������=������ͬ����
��M��N����ʱ��ѧ��Ӧ�٣�v��N����v��M����
��Ϊ���CO2��ת���ʳ��ɸı��¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ������$\frac{n��H{\;}_{2}��}{n��CO{\;}_{2}��}$��ֵ
��ͼ��M��ʱ���������Ϊ10L����N���Ӧ��ƽ�ⳣ��K=1.04������ֵ����2λС����
��3��һ�������£����ݻ������ij�ܱ������м���a mol CO2��b mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ʹ������Ӧ������CO2���������Ϊ�㶨ֵ��a��b�Ĺ�ϵ��a=b��
���� ��1����֪����CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ•mol-1��
��CO��g��+2H2��g��?CH3OH ��g����H2=-90.1kJ•mol-1��
��2CH4��g��+O2��g��?2CH3OH��g����H3��
��˹���ɣ���֪�١�2+�ڡ�2=�ۣ��ʷ�Ӧ��Ҳ������Ӧ���㣻
��2������ͼ��֪��ѹǿһ��ʱ���¶�Խ�ߣ�CH3OH�����ʵ���ԽС��˵�������¶�ƽ�����淴Ӧ�����ƶ���
������ӦΪ���������С�ķ�Ӧ���¶�һ��ʱ������ѹǿ��ƽ��������Ӧ�����ƶ����״������ʵ��������ͼ��֪��P1��P2������ѹǿ�Է�Ӧ���ʵ�Ӱ����⣻
���ڿ��淴Ӧ�У�����һ�ַ�Ӧ��Ũ�ȿ��������һ��Ӧ���ת���ʣ�
��ͼ��M��ʱ���������Ϊ10L�����M��������֪��M���CH3OH�����ʵ���Ϊ0.25mol��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L�� 0.1 0.275 0 0
ת����mol/L��0.025 0.075 0.025 0.025
ƽ�⣨mol/L�� 0.075 0.2 0.025 0.025
����K=$\frac{c��H{\;}_{2}O��c��CH{\;}_{3}OH��}{{c}^{3}��H{\;}_{2}��c��CO{\;}_{2}��}$���㣻
��3����ת���Ķ�����̼�����ʵ���Ϊx��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����
��ʼ a b 0 0
ת�� x 3x x x
ƽ�� a-x b-3x x x
��CO2���������Ϊ$\frac{a-x}{a-x+b-3x+x+x}$=$\frac{a-x}{a+b-2x}$���ݴ����ۣ�
��� �⣺��1����֪����CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g����H1=-35.4kJ•mol-1��
��CO��g��+2H2��g��?CH3OH ��g����H2=-90.1kJ•mol-1��
��2CH4��g��+O2��g��?2CH3OH��g����H3��
��˹���ɣ���֪�١�2+�ڡ�2=�ۣ��ʡ�H3=2��H1+2��H2=2����-35.4kJ•mol-1-90.1kJ•mol-1��=-251kJ•mol-1��
�ʴ�Ϊ��251��
��2������ͼ��֪��ѹǿһ��ʱ���¶�Խ�ߣ�CH3OH�����ʵ���ԽС��˵�������¶�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
������ӦΪ���������С�ķ�Ӧ���¶�һ��ʱ������ѹǿ��ƽ��������Ӧ�����ƶ����״������ʵ�������ѹǿP1��P2��ѹǿ����Ӧ����Ҳ��������v��N����v��M����
�ʴ�Ϊ������
���ڿ��淴Ӧ�У�����һ�ַ�Ӧ��Ũ�ȿ��������һ��Ӧ���ת���ʣ�����Ϊ���CO2��ת���ʳ��ɸı��¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ������$\frac{n��H{\;}_{2}��}{n��CO{\;}_{2}��}$��ֵ��
�ʴ�Ϊ������$\frac{n��H{\;}_{2}��}{n��CO{\;}_{2}��}$��ֵ��
��ͼ��M��ʱ���������Ϊ10L�����M��������֪��M���CH3OH�����ʵ���Ϊ0.25mol��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L�� 0.1 0.275 0 0
ת����mol/L��0.025 0.075 0.025 0.025
ƽ�⣨mol/L�� 0.075 0.2 0.025 0.025
K=$\frac{c��H{\;}_{2}O��c��CH{\;}_{3}OH��}{{c}^{3}��H{\;}_{2}��c��CO{\;}_{2}��}$=$\frac{0.025��0.025}{0��{2}^{3}��0.075}$=1.04��
�ʴ�Ϊ��1.04��
��3����ת���Ķ�����̼�����ʵ���Ϊx��
���ݷ�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����
��ʼ a b 0 0
ת�� x 3x x x
ƽ�� a-x b-3x x x
��CO2���������Ϊ$\frac{a-x}{a-x+b-3x+x+x}$=$\frac{a-x}{a+b-2x}$��Ҫʹ$\frac{a-x}{a+b-2x}$Ϊ�㶨��ֵ����a=b��
�ʴ�Ϊ��a=b��
���� ��������ƴ������Ŀ���漰ԭ��ء���Ӧ�ȼ��㡢��ѧƽ�ⳣ������ѧƽ��Ӱ�����ء��л����ƶϵȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | CH3CH�TCH2+Br2��ˮ��Һ��-��CH2BrCH�TCH2+HBr | |
| B�� | nCH3CH�TCH2$\stackrel{һ��������}{��}$[CH3-CH-CH2]n | |
| C�� | CH2�TCH-CH�TCH2+Br2-��CH2Br2-CH�TCH-CH3 | |
| D�� | CH3CH2OH$\stackrel{ŨH_{2}SO_{4}}{��}$CH2�TCH2��+H2O |
| A�� | ������������������ | |
| B�� | �����ʵ�Ħ��������160g•mol-1 | |
| C�� | �����ʵļ��Բ�ǿ | |
| D�� | ��1molNaAl��OH��2CO3��������1mol•L-1HCl��ʱ�����ռ���2.5molCO2 |
��1���õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ��
5 C+4 KMnO4+6 H2SO4��5CO2��+4MnSO4+2K2SO4+6H2O��
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b ��ֵ��1 �������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���10molCO��5molH2O��2molCO2��5molH2�����ʱV����V�������������������=������
��1����֪��N2��g��+2O2��g���T2NO2��g����H=+67.7kJ•mol-1
N2H4��g��+O2��g���TN2��g��+2H2O��g����H=-534.0kJ•mol-1
2NO2��g��?N2O4��g����H=-52.7kJ•mol-1����̬����N2O4��ȼ�����ɵ�����ˮ�������Ȼ�ѧ����ʽΪ
2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-1083.0kJ•mol-1��
��2����ҵ�Ͽ��ô�������������İ���Ӧ�Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪNaClO+2NH3=NaCl+N2H4+H2O��
��3��һ�������£���2L�ܱ������г���3.2mol NH3��4.4mol O2��������Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H��0�����ƽ��ʱ���������ʾ��
���� ���ʵ���/mol �¶�/�� | NO | H2O |
| T1 | 1.6 | 2.4 |
| T2 | 1.2 | 1.8 |
��T1�� T2 �����������������=������
�����д�ʩ�У��������NH3��ת���ʣ����ܼӿ췴Ӧ���ʵ���d����ѡ����ĸ����
a�������¶� b�������¶� c������������� d�����������������ٳ���һ����O2
��4�������˺���������̬ϵͳ�У������õ绯ѧװ�÷�����Ӧ��2CO2�T2CO+O2���ֽ��ȥCO2���ṩ�����O2����֪�÷�Ӧ��������ӦʽΪ4OH--4e-�T2H2O+O2������������ӦʽΪ2CO2+4e-+2H2O=2CO+4OH-�����������������Ʒ�Ӧ��2CO��g��=2C��s��+O2��g����H��0��������CO����Ⱦ�����ж���������Ƿ��������ǡ����������Ǹ÷�ӦΪ��H��0����S��0�ķ�Ӧ�������Է����У�
| ��Ӧ���� | ��ѧ����ʽ | �ʱ��H��kJ/mol�� | ���Ea��kJ/mol�� |
| �������� | CH4��g��+2O2��g��=CO2��g��+2H2O��g�� | -802.6 | 125.6 |
| CH4��g��+O2��g��=CO2��g��+2H2��g�� | -322.0 | 172.5 | |
| �������� | CH4��g��+H2O��g��=CO��g��+3H2��g�� | 206.2 | 240.1 |
| CH4��g��+2H2O��g��=CO2��g��+4H2��g�� | 165.0 | 243.9 |
��1����ӦCO��g��+H2O��g���TCO2��g��+H2��g���ġ�H=-41.2kJ•mol-1��
��2���ڳ�ʼ�Σ��������������ķ�Ӧ����С�ڼ��������ķ�Ӧ���ʣ�����ڡ�����С�ڡ����ڡ�����
��3���������෴Ӧ����ij��֣�B����ƽ��ѹǿ��pB���������ʵ���Ũ�ȣ�cB��Ҳ�ɱ�ʾƽ�ⳣ��������Kp������ӦCH4��g��+H2O��g��?CO��g��+3H2��g����Kp=$\frac{{p}^{3}��{H}_{2}����p��CO��}{p��C{H}_{4}����p��{H}_{2}O��}$�� �����¶ȵ����ߣ���ƽ�ⳣ���������������С�����䡱����
��4���������Ƕȷ������������������������Ƚ�֮�����ڷ��ȵļ���������ӦΪ���ȵ����������ṩ������
��5����ijһ�������ϱȵ�����£��¶ȡ�ѹǿ��H2��CO���ʵ���������Ӱ������ͼ��
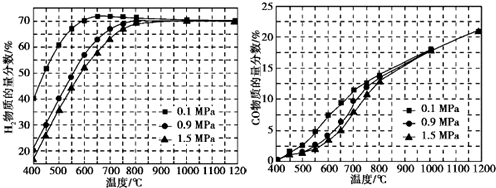
����Ҫ�ﵽH2���ʵ���������65%��CO���ʵ���������10%����������������ʵ���B��
A.600�棬0.9MPa B.700�棬0.9Mpa
C.800�棬1.5MPa D.1 000�棬1.5MPa
�ڻ���600�棬0.1MPa�����£�ϵͳ��H2���ʵ��������淴Ӧʱ�䣨�ӳ��½��Ͽ�ʼ��ʱ���ı仯����ʾ��ͼ��
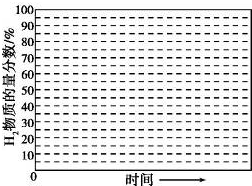
��6������������������������յ���H2���ʵ����������ͣ�ԭ���ǵ������ɵ�������������Ӧ��