��Ŀ����
12��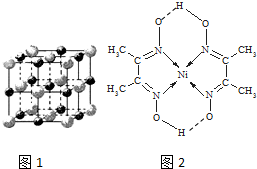 ��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��| ������/kJ•mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
��2�������Ĺ��������е�����ԼΪ399kJ•mol-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ���������е������ȵ����ʷ�������Ҫ�Ļ�ѧ��C-C��C-N��C-S�ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ�
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ•mol-1 | 347 | 305 | 259 |
��3��ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ���ͼ1��ʾ��������3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ•mol-1 | 786 | 715 | 3401 |
��4��ij�����ķ��ӽṹ��ͼ2��ʾ��������ڲ�����AC������ţ���
A�����Ӽ� B�����Լ� C�������� D����λ�� E����� F���Ǽ��Լ�
��5��Ϊ��������ЧӦ��Ӱ�죬��ѧ����Ʒ�Ӧ��CO2+4H2��CH4+2H2O�Լ�С������CO2������1mol CH4���ɣ�����6mol�Ҽ���2mol�м����ѣ�
���� ��1���ɵ����ܿ�֪��A��ʧȥ3�����ӣ�B��ʧȥ2�����ӣ���AΪAl��BΪMg��
��2�����������Ĺ��������е������뵰���ʷ�������Ҫ��ѧ���������������ȽϷ�������İ�����Ϊ�ʰ��
��3�����Ӿ����о�����Խ���γɵ����Ӿ���Խ�ȶ����۵�Խ��Ӳ��Խ�����������ӵİ뾶������йأ����Խ�ࡢ���Ӱ뾶ԽС��������Խ���ݽṹ���������ж�һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+������
��4������ͼƬ�������еļ���
��5�������ж�CO2��H2�����к��ж��٦ļ��ͦм������ݻ�ѧ����ʽ���㣮
��� �⣺��1���ɵ����ܿ�֪��A��ʧȥ3�����ӣ�����ϼ�Ϊ+3�ۣ�B��ʧȥ2�����ӣ�����ϼ�Ϊ+2�ۣ���AΪAl��BΪMg��ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����縺��A��B��
�ʴ�Ϊ��+3������
��2������Ϊ300nm�������Ĺ��������е�����ԼΪ399kJ/mol���ȵ����ʷ�����C-C��C-N��C-S�ļ��ܶ������Բ���Ϊ300nm�������Ĺ������ƻ������ʷ����еĻ�ѧ�����Ӷ��ƻ������ʷ��ӣ���İ�����Ϊ�ʰ��ᣬ�ʰ������Ȼ���̼ԭ��Ϊ sp2 �ӻ�����һ��̼ԭ��Ϊ sp3 �ӻ���
�ʴ�Ϊ���������е������ȵ����ʷ�������Ҫ�Ļ�ѧ��C-C��C-N��C-S�ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ�sp2��sp3��
��3�����Ӿ����о�����Խ���γɵ����Ӿ���Խ�ȶ����۵�Խ��Ӳ��Խ�����������ӵİ뾶������йأ����Խ�ࡢ���Ӱ뾶ԽС��������Խ��TiN�����������������Ϊ3��������������������ɣ�MgO��CaO�����������ͬ����þ���Ӱ뾶С�ڸ����Ӱ뾶���Ȼ��������������������Ϊ1���Ҽ����Ӱ뾶�������Ӱ뾶�������Ӱ뾶���������Ӱ뾶������KCl��MgO��CaO��TiN4�����Ӿ����۵�Ӹߵ��͵�˳����TiN��MgO��CaO��KCl��
MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ�����һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+����Ϊ12��
�ʴ�Ϊ��TiN��MgO��CaO��KCl��12��
��4����������д��ڵĻ�ѧ���У��ǽ���Ԫ��֮��Ĺ��ۼ�����Ԫ���뵪Ԫ��֮�����λ������ԭ�Ӻ���ԭ��֮����������ѡAC��
��5��1��CO2��4��H2�����й�����6���ļ���2�м�������1molCH4���ɣ�����6mol�ļ���2mol�м����ѣ��ʴ�Ϊ��6��2��
���� ���⿼���˻��ϼۺ͵縺�Ե��жϡ���ѧ����֪ʶ�㣬ע�⣨3������Ľṹ��ע�⾧���������Ӿ����۵�Ĺ�ϵ����Ŀ�Ѷ��еȣ�

 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�| A�� | Y��Z��W����Ԫ����ɻ������ˮ��Һ�����Լ��� | |
| B�� | Z��Y��Z��W�γɵĻ�����Ļ�ѧ��������ȫ��ͬ | |
| C�� | �����ӵİ뾶��Z��R��W��Y | |
| D�� | ��ǽ�����Y��R������X��Y��ɻ�����ķе����X��R��ɵĻ����� |
| A�� | 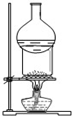 ����I2��NH4Cl�����ķ��� | B�� |  ��������ȡ���۲�Fe��OH��2 | ||
| C�� |  ��֤����������ˮ | D�� |  �������ſ������ռ�CO2���� |
| A�� | Ca2+��K+��Cl-��NO3- | B�� | Ag+��Na+��NO3-��Cl- | ||
| C�� | Cu2+��K+��SO42-��Cl- | D�� | Ba2+��NH4+��SO42-��Cl- |
| A�� | �Ȼ�þ���ڵ���ʣ���ҵ�Ͽ��õ������Һ�ķ�����þ | |
| B�� | �ƼغϽ��Ӳ��С�������������ӷ�Ӧ�ѵ��Ƚ����� | |
| C�� | Fe2O3�Ǻ���ɫ���壬�����Ƴɺ�ɫ�����ᡢͿ�� | |
| D�� | �嵥����CCl4�е��ܽ�ȴ���CCl4��ȡBr- |
 ��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ�Ϊ I1=738��I2=1451��I3=7733��I4=10540����λ��kJ/mol�� |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���3������ԭ�ӹ���ʷĴ����Σ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
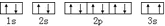 ��ͬѧ�����ĵ����Ų�ͼΥ��������ԭ����
��ͬѧ�����ĵ����Ų�ͼΥ��������ԭ������4��Gλ�ڵڢ�B��d�����۵����Ų�ʽΪ3d54s2��
��5��DE3����ԭ�ӵ��ӻ���ʽΪsp3���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ�����Σ�
��6��FԪ�صľ�����ͼ��ʾ������þ������ܶ�Ϊa g/cm3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM g/mol����Fԭ�ӵİ뾶Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{2M}{a{N}_{A}}}$ cm��
| A�� | ��Ag2S��Һ�еμ�����ϡNaCl��Һ����ɫ������ɰ�ɫ��Ag2S+Cl-?AgCl+S2- | |
| B�� | ��K2Cr2O7��Һ�еμ�����ŨH2SO4����Һ��Ϊ��ɫ��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+ | |
| C�� | ����۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ������4H++4I-+O2=2I2+2H2O | |
| D�� | ��ˮ���ᣨ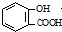 ���еμ�NaHCO3��Һ���ų���ɫ���壺 ���еμ�NaHCO3��Һ���ų���ɫ���壺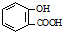 +2HCO3-�� +2HCO3-��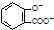 +2CO2��+2H2O +2CO2��+2H2O |
| A�� | 2��3 | B�� | 1��3 | C�� | 4��3 | D�� | 3��2 |
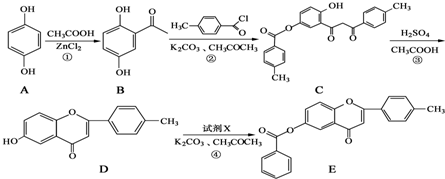
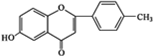 +
+ $\stackrel{K_{2}CO_{3}��CH_{3}COCH_{3}}{��}$
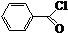
$\stackrel{K_{2}CO_{3}��CH_{3}COCH_{3}}{��}$ +HCl��
+HCl��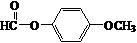 ��
��