��Ŀ����
14��X��Y��Z��R��W��ԭ������������������ֶ�����Ԫ�أ�Y��Rͬ���壬����ɹ��ۻ�����RY2��Y��Z����������֮����W��������������ͬ��25��ʱ��0.1mol/L X��W�γɻ������ˮ��ҺpHΪ1������˵����ȷ���ǣ�������| A�� | Y��Z��W����Ԫ����ɻ������ˮ��Һ�����Լ��� | |
| B�� | Z��Y��Z��W�γɵĻ�����Ļ�ѧ��������ȫ��ͬ | |
| C�� | �����ӵİ뾶��Z��R��W��Y | |
| D�� | ��ǽ�����Y��R������X��Y��ɻ�����ķе����X��R��ɵĻ����� |
���� X��Y��Z��R��W��ԭ������������������ֶ�����Ԫ�أ�Y��Rͬ���壬Y���ڵڶ����ڣ�R���ڵ������ڣ�����ɹ��ۻ�����RY2����YΪ��Ԫ�أ�RΪ��Ԫ�أ�RY2ΪSO2��W��ԭ������������Ԫ�أ���WΪClԪ�أ�Y��Z����������֮����W��������������ͬ����Z������������Ϊ7-6=1�����ڵڢ�A�壬ԭ������������Ԫ�أ���ZΪNaԪ�أ�25��ʱ0.1mol•L-1X��W�γɻ������ˮ��ҺpHΪ1������ǿ�ᣬ��XΪ��Ԫ�أ��ݴ˽��
��� �⣺X��Y��Z��R��W��ԭ������������������ֶ�����Ԫ�أ�Y��Rͬ���壬Y���ڵڶ����ڣ�R���ڵ������ڣ�����ɹ��ۻ�����RY2����YΪ��Ԫ�أ�RΪ��Ԫ�أ�RY2ΪSO2��W��ԭ������������Ԫ�أ���WΪClԪ�أ�Y��Z����������֮����W��������������ͬ����Z������������Ϊ7-6=1�����ڵڢ�A�壬ԭ������������Ԫ�أ���ZΪNaԪ�أ�25��ʱ0.1mol•L-1X��W�γɻ������ˮ��ҺpHΪ1������ǿ�ᣬ��XΪ��Ԫ�أ�
A��Y��Z��W����Ԫ����ɵĻ�������NaClO�ȣ�NaClO��Һ�д������ˮ�⣬��Һ�Լ��ԣ���A��ȷ��
B��Z��Y�����γ�Na2O��Na2O2��ǰ�ߺ������Ӽ������ߺ������Ӽ������ۼ�����Z��W�γɵĻ�����ΪNaCl��ֻ�������Ӽ�����B����
C��Y��Z��R��W�ļ����ӷֱ�ΪO2-��Na+��S2-��Cl-�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��S2-��Cl-��O2-��Na+����C����
D��X��Y��ɻ�����ΪH2O��H2O2��X��R��ɵĻ�����H2S��ǰ�߷��Ӽ����������е�������⣬��D����
��ѡB��
���� ���⿼��λ�ýṹ���ʹ�ϵ���Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ�����Y��RԪ�ص�λ�ü������γɵĹ��ۻ�����RY2�����ƶϵ�ͻ�ƿڣ�ע��������������ʵ�Ӱ�죮

| A�� | Cl- | B�� | Al3+ | C�� | H+ | D�� | OH- |
| A�� | 1 L 1 mol•L-1��NaClO��Һ�к���ClO-����ĿΪNA | |
| B�� | 78 g������C=C������ĿΪ3 NA | |
| C�� | ������ĿΪ0.1NA��N2��NH3������壬ԭ�Ӽ京�еĹ��õ��Ӷ���ĿΪ0.3NA | |
| D�� | 1 mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2 NA |
| A�� | ̼����������Ӧ��������ʳƷ�����ɼ� | |
| B�� | �ƾ��к�ǿ�Ļ�ԭ�ԣ���������TiCl4��Ӧ��ȡ�� | |
| C�� | Al2O3���кܸߵ��۵㣬���������������ռ������ | |
| D�� | ͭ�Ľ��������Ա���ǿ�������ڱ���ʯ�ܵ���̼�ظֹܣ��Լ����丯ʴ |

| A�� | ���������������Եó���ɫ��Һ��n��Cu2+��=0.02 mol | |
| B�� | ��������������ٵĹ�������һ����Fe2O3 | |
| C�� | ������м��ٵ�3 g����һ���ǻ���� | |
| D�� | ���ݲ��������ж�X������������������Ϊ50% |
| A�� | ��ϵͳ���������л��� 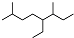 ������Ϊ3��7-����-4-�һ����� ������Ϊ3��7-����-4-�һ����� | |
| B�� | ���������������е�ԭ�ӿ��ܹ�ƽ�� | |
| C�� | �Ȱ�����ӣ���-��������ᣩ����ֻ���γ�1�ֶ��ģ������������칹�� | |
| D�� | ��һ�������£�1.0 mol�� 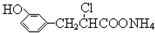 ������뺬3.0 mol NaOH����Һ��ȫ��Ӧ ������뺬3.0 mol NaOH����Һ��ȫ��Ӧ |
| A�� | Ϊ��С����к͵ζ���ʵ������ƿ����ϴ������ɺ�ʹ�� | |
| B�� | �ڰ�ˮ�м���������ˮ���Ȼ�粒������ʹ��Һ�е�c��H+������ | |
| C�� | ��֪NaAlO2��Һ��pH��7���������ɲ����պ�õ��IJ�����ΪNaAlO2 | |
| D�� | ��H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ•mol-1����֪100 mL 0.1 mol•L-1 ��������100 mL 0.1 mol•L-1 �İ�ˮ��ϣ��ų�����������0.573 kJ |
 ��ҵ�ϣ���ǿ�����������õ�ⷨ��ȥ��ˮ�е�CN-��װ������ͼ��ʾ�����η����ķ�Ӧ�У�
��ҵ�ϣ���ǿ�����������õ�ⷨ��ȥ��ˮ�е�CN-��װ������ͼ��ʾ�����η����ķ�Ӧ�У���CN--2e-+2OH-�TCNO-+H2O
��2Cl-һ2e-�TCl2��
��3Cl2+2CNO-+8OH-�TN2+6Cl-+2CO32-+4H2O
����˵����ȷ���ǣ�������
| A�� | ��ȥ1 mol CN-�����·������ת��5 mol���� | |
| B�� | ͨ���������Һ��pH�������� | |
| C�� | Ϊ��ʹ����������������Ҫ���ϲ���NaCl | |
| D�� | ���缫�Ϸ����ķ�ӦΪFeһ2eһ�TFe2+ |
 ��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��| ������/kJ•mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
��2�������Ĺ��������е�����ԼΪ399kJ•mol-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ���������е������ȵ����ʷ�������Ҫ�Ļ�ѧ��C-C��C-N��C-S�ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ�
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ•mol-1 | 347 | 305 | 259 |
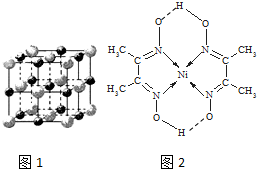
��3��ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ���ͼ1��ʾ��������3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ•mol-1 | 786 | 715 | 3401 |
��4��ij�����ķ��ӽṹ��ͼ2��ʾ��������ڲ�����AC������ţ���
A�����Ӽ� B�����Լ� C�������� D����λ�� E����� F���Ǽ��Լ�
��5��Ϊ��������ЧӦ��Ӱ�죬��ѧ����Ʒ�Ӧ��CO2+4H2��CH4+2H2O�Լ�С������CO2������1mol CH4���ɣ�����6mol�Ҽ���2mol�м����ѣ�