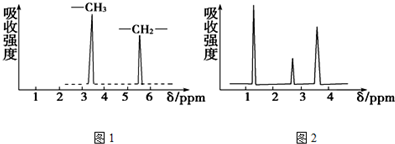
��Ŀ����
10����ʵ���п�����ͼ��ʾװ���Ʊ�1��2-�������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ�壨���渲������ˮ����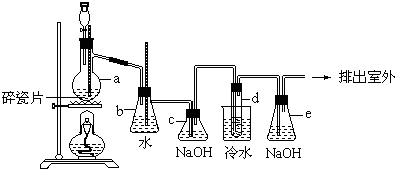
��д���пհף�
��1��д���������Ʊ�1��2-���������������ѧ����ʽCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��CH2=CH2+Br2��CH2BrCH2Br��
��2������c��NaOH��Һ�������ǣ���ȥ��ϩ�д�����SO2��CO2���������壻
��3��ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û�����⣬����ԭ������ϩ��������ͨ��Һ�壩�ٶȹ��죨��ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棬��������Ӧ������˵��һ����
��4����Ӧ��Ũ�������������ˮ�� �����ã�
���� ʵ��ԭ�����Ҵ���Ũ���ᷴӦ��Ҫ������ϩ���壬��ӦΪ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O�������ܻ���̼��Ũ���ᷴӦ���ɵ�CO2��SO2���壬bΪ��ȫƿ��cΪ��������װ�ã�dΪ��ϩ����ķ�Ӧװ�ã���ӦΪ��CH2=CH2+Br2��CH2BrCH2Br��eΪβ������װ�ã�
��1��ʵ���������Ҵ���Ũ�����������ȡ��ϩ��Ȼ������ϩ���嵥�ʵļӳɷ�Ӧ���Ƶ�1��2-�������飻
��2����������ķ�����֪����ϩ�п�����CO2��SO2���������壬��ʵ���и��ţ�����Ҫ��ȥ��CΪ����װ�ã��������ͨ��cƿ��CO2��SO2���屻�����������գ��Է�ֹ���������巴Ӧ��
��3���Ҵ���Ũ������Һû����ȫ��Ӧ������ϩ���Լ���ϩ���巴Ӧ�������ʼ��ٵĿ���ԭ����н��
��4��Ũ���������ˮ�ԣ���ˮ�ԣ�ǿ�����ԣ���ϸ�ʵ�鷴Ӧ���
��� �⣺��1��ʵ���������Ҵ���Ũ�����������ȡ��ϩ��Ȼ������ϩ���嵥�ʵļӳɷ�Ӧ���Ƶ�1��2-�������飬������Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��CH2=CH2+Br2��CH2BrCH2Br��
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��CH2=CH2+Br2��CH2BrCH2Br��
��2��a��Ũ�������ǿ�����ԣ����Ҵ������ɶ�����̼����������ԭ�ɶ�������CH3CH2OH+4H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$4SO2��+CO2��+7H2O+C��������̼�����������ܺ�����������Һ��Ӧ�������գ���ӦΪ��SO2+2NaOH=Na2SO3+H2O��CO2+2NaOH�TNa2CO3+H2O����������c����װ��NaOH��Һ����Ϊ����ȥ��ϩ�д�����SO2��CO2�������������ʣ��Է�ֹ���������巴Ӧ��
�ʴ�Ϊ��NaOH��Һ����ȥ��ϩ�д�����SO2��CO2���������壻
��3������ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³��������ԭ���������ϩ��������ͨ��Һ�壩�ٶȹ��죬���´���ϩû�к��巢����Ӧ�����⣬Ҳ������ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170������������ѵȣ�
�ʴ�Ϊ����ϩ��������ͨ��Һ�壩�ٶȹ��죨��ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棬��������Ӧ����
��4�������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ��Ũ������������á���ˮ�����ã�
�ʴ�Ϊ����������ˮ����
���� ���⿼�����Ʊ�ʵ�鷽������ơ����������ȡ��������Ŀ�Ѷ��еȣ�ע���������������ȡԭ������Ӧװ��ѡ���ӡ��ᴿ�����ǽ���Ĺؼ���ע������ѧ���������⡢��������������

| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | ||
| X2 | Y2 | XY3 | ||
| �� | 1 | 3 | 0 | ����46.3 kJ |
| �� | 0.8 | 2.4 | 0.4 | Q��Q��0�� |
| A�� | �����١����з�Ӧ��ƽ��ʱXY3��ƽ��Ũ����ͬ | |
| B�� | �����١����дﵽƽ��ʱ�����ʵİٷֺ�����ͬ | |
| C�� | ��ƽ��ʱ������������XY3�����ʵ���Ũ�Ⱦ�Ϊ2 mol•L-1 | |
| D�� | �����������Ϊ0.20 L�����ƽ��ʱ�ų�����������46.3 kJ |
R-OH+HBr?R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȣ��й����������
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g•cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��1�����������1-�嶡����Ʊ�ʵ���У���������������õ�����b��
a��Բ����ƿ b�� ����ƿ c����ƿ d����Ͳ
��2���������ˮ����С�ڣ�����ڡ��������ڡ���С�ڡ�����Ӧ�Ĵ�����ԭ���Ǵ����ӿ���ˮ����֮���γ�����������������ˮ����֮���γ������
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã��������²㣨��ϲ㡱�����²㡱���ֲ㡱����
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ�IJ���ȷ����a��
a�� ˮ�Ƿ�Ӧ�Ĵ��� b������Br2������ c������HBr�Ļӷ� d�����ٸ�����ϩ���ѵ�����
��5�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������ԭ����ƽ��������������ķ����ƶ�����Ӧ�������ƶ����������Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ����1-�嶡�����������ķе���С�����߷�Ӧ�������н϶����������������
| A�� | ������Һ�е�pHǰ�ߴ� | |
| B�� | ���߽�����ˮ��ƽ�⣬�����ڵ���ƽ�� | |
| C�� | ����Һ�о�����c��Na+��+c��H+��=c��HCO${\;}_{3}^{-}$��+2c��CO${\;}_{3}^{2-}$��+c��OH-�� | |
| D�� | �ֱ����NaOH���壬�ָ���ԭ�¶ȣ�c��CO${\;}_{3}^{2-}$��ǰ���������С |
| A�� | �������̿�ʯ��MnO2����������Ϊ75% | |
| B�� | ��������HCl�����ʵ���Ϊ4mol | |
| C�� | �μӷ�Ӧ��HCl������Ϊ146g | |
| D�� | ����ԭ��MnO2�����ʵ���Ϊ1mol |