��Ŀ����
[��ѧ����ѡ��ѧ�뼼��]��15�֣�
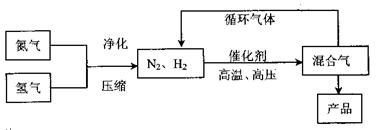
��ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2��g��+3H2��g�� 2NH3��g������
2NH3��g������
���ֹ�ҵ�������£�
�ش��������⣺
��1����֪��N2��g��+O2��g��=2NO��g�� ��H=180.5kJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g�� ��H=-905kJ/mol
2H2��g��+O2��g��=2H2��g�� ��H=-483.6kJ/mol
��N2��g��+2H2 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ��
��2�������ҵ�ϣ���һ���¶��£���1.5molN2�����6molH2����ͨ�뵽���Ϊ1�����ܱ������С�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ�ⳣ��Ϊ ���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ��������� ;
������ѹǿ ������Ӧ���ʵ�Ũ��
��ʹ�ô��� �ܽ����¶�
��3���ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ��ȡ����ѭ�������̡�����Ӧ��ͨ�����ͻ��������¶ȶ�ʹ����������������ַ������ʵķ�����ԭ���������������ַ�
���� �����ţ�
�ٹ��� ������ ������ ����ȡ
������ ;
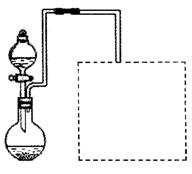
��4���������������������Ͱ����Ĺܵ��Ƿ�©�������©������а��̣��ɷ�Ϊ�Ȼ�泥����ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5������ó�������ˮ�����ʵ���Ũ��Ϊ20mol/L��ʵ����������80mLŨ��Ϊ5mol/L�İ�ˮʱ����ȡ20mol/L�İ�ˮ mL����100mL������ƿ��������ð�ˮ��pH=a��������ͬ���������ʱ����Һ�����ԣ���������pH 14-a������ڡ���С�ڡ����ڡ�����
��ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2��g��+3H2��g��
 2NH3��g������
2NH3��g���������ֹ�ҵ�������£�

�ش��������⣺
��1����֪��N2��g��+O2��g��=2NO��g�� ��H=180.5kJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g�� ��H=-905kJ/mol
2H2��g��+O2��g��=2H2��g�� ��H=-483.6kJ/mol
��N2��g��+2H2
 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ����2�������ҵ�ϣ���һ���¶��£���1.5molN2�����6molH2����ͨ�뵽���Ϊ1�����ܱ������С�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ�ⳣ��Ϊ ���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ��������� ;
������ѹǿ ������Ӧ���ʵ�Ũ��
��ʹ�ô��� �ܽ����¶�
��3���ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ��ȡ����ѭ�������̡�����Ӧ��ͨ�����ͻ��������¶ȶ�ʹ����������������ַ������ʵķ�����ԭ���������������ַ�
���� �����ţ�
�ٹ��� ������ ������ ����ȡ
������ ;
��4���������������������Ͱ����Ĺܵ��Ƿ�©�������©������а��̣��ɷ�Ϊ�Ȼ�泥����ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5������ó�������ˮ�����ʵ���Ũ��Ϊ20mol/L��ʵ����������80mLŨ��Ϊ5mol/L�İ�ˮʱ����ȡ20mol/L�İ�ˮ mL����100mL������ƿ��������ð�ˮ��pH=a��������ͬ���������ʱ����Һ�����ԣ���������pH 14-a������ڡ���С�ڡ����ڡ�����
��1��-92.4kJ/mol
��2��0.057��mol��L-1��2���٢�
��3���ڣ�ͨ���¶ȵĸı���ı����ʵ�״̬���ﵽ�����Ŀ�ġ������û��̸��״̬�ĸı䣬��ֻ˵�¶Ȳ��÷֣���
��4��8NH3+3Cl2=N2+6NH4Cl
��5��25mL����
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
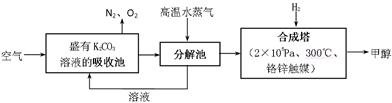
 8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ ��
8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ �� 2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ�����
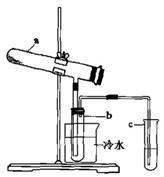
2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ����� Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ�
Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ� ��
��
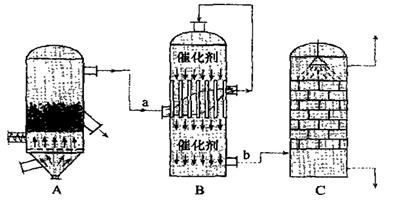
 Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת����
Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� ���� ���� ���ɲ���������
���� ���� ���ɲ���������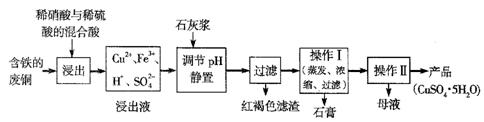
 14
14