��Ŀ����
�������̣�MnO2����Ũ�����ϼ��ȿɵõ��������Ȼ��̵ȣ���ͼ����ȡ��̽��Cl2��ѧ���ʵ�װ��ͼ��
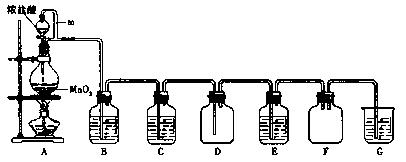
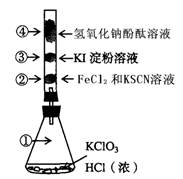
��1��д��Բ����ƿ�з�Ӧ�Ļ�ѧ����ʽ ��
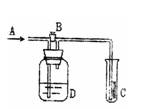
��2��A��m�ܵ������� ��װ��F�������� ��
��3����Ҫ�õ����﴿�������壬��B��Ӧʢ�ŵ��Լ��� ��C��Ӧʢ�ŵ��Լ��� ��
��
��4��E����װ��FeCl2��Һ����Ӧ�����ӷ���ʽΪ ��E����װ�е��۵⻯����Һ���ܹ۲쵽��ʵ�������� ��
��5��ʵ���з��֣�Ũ������MnO2��ϼ�������������ϡ������MnO2��ϼ��Ȳ����������������������ѧС��ԡ�Ӱ���������ɵ�ԭ���������ۣ������������ʵ�鷽����
a��ϡ�������MnO2�У�Ȼ��ͨ��HCl���壬����
 b��ϡ�������MnO2�У�Ȼ�����NaCl���壬����
b��ϡ�������MnO2�У�Ȼ�����NaCl���壬����
c��ϡ�������MnO2�У�Ȼ�����Ũ���ᣬ����
d��MnO2��NaCl��Ũ��Һ��ϣ�����
e��Ũ������NaCl���塢MnO2���干��
��ʵ��b��Ŀ���� ��ʵ��c��Ŀ���� ��
��ʵ������a��c��e�л���ɫ�������ɣ�b��dû�л���ɫ�������ɡ��ɴ˵ó�Ӱ���������ɵ�ԭ���� ��

��1��д��Բ����ƿ�з�Ӧ�Ļ�ѧ����ʽ ��
��2��A��m�ܵ������� ��װ��F�������� ��
��3����Ҫ�õ����﴿�������壬��B��Ӧʢ�ŵ��Լ��� ��C��Ӧʢ�ŵ��Լ���
 ��
����4��E����װ��FeCl2��Һ����Ӧ�����ӷ���ʽΪ ��E����װ�е��۵⻯����Һ���ܹ۲쵽��ʵ�������� ��
��5��ʵ���з��֣�Ũ������MnO2��ϼ�������������ϡ������MnO2��ϼ��Ȳ����������������������ѧС��ԡ�Ӱ���������ɵ�ԭ���������ۣ������������ʵ�鷽����
a��ϡ�������MnO2�У�Ȼ��ͨ��HCl���壬����
 b��ϡ�������MnO2�У�Ȼ�����NaCl���壬����
b��ϡ�������MnO2�У�Ȼ�����NaCl���壬����c��ϡ�������MnO2�У�Ȼ�����Ũ���ᣬ����
d��MnO2��NaCl��Ũ��Һ��ϣ�����
e��Ũ������NaCl���塢MnO2���干��
��ʵ��b��Ŀ���� ��ʵ��c��Ŀ���� ��
��ʵ������a��c��e�л���ɫ�������ɣ�b��dû�л���ɫ�������ɡ��ɴ˵ó�Ӱ���������ɵ�ԭ���� ��
��1��MnO2+4HCl=MnCl2+Cl2��+2H2O ��2�֣�
��2��ƽ����ѹ��ʹ��Һ©���е�Һ��˳���µΣ�2�֣���
��ֹG��Һ�嵹����E�У�2�֣�
��3������ʳ��ˮ������NaCl��Һ�� ��2�֣� �� Ũ���ᣨ2�֣�
��4��2Fe2++Cl2=2Fe3++2Cl- ��2�֣��� ��ɫ��Һ����ɫ��2�֣�
��5����̽��Cl-Ũ�ȵ�Ӱ�죨1�֣��� ̽��H+Ũ�ȵ�Ӱ�죨1�֣�
��H+Ũ�ȵĴ�С��1�֣�
��2��ƽ����ѹ��ʹ��Һ©���е�Һ��˳���µΣ�2�֣���
��ֹG��Һ�嵹����E�У�2�֣�
��3������ʳ��ˮ������NaCl��Һ�� ��2�֣� �� Ũ���ᣨ2�֣�
��4��2Fe2++Cl2=2Fe3++2Cl- ��2�֣��� ��ɫ��Һ����ɫ��2�֣�
��5����̽��Cl-Ũ�ȵ�Ӱ�죨1�֣��� ̽��H+Ũ�ȵ�Ӱ�죨1�֣�
��H+Ũ�ȵĴ�С��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ