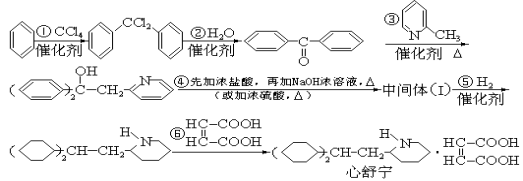
��Ŀ����
����Ŀ����1���״�����Ҫ�Ļ���ԭ�ϣ��ֿ���ȼ�ϡ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�����Ҫ��Ӧ���£�
��CO��g��+2H2��g��![]() CH3OH��g�� ��H1
CH3OH��g�� ��H1
��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g�� ��H2= ��58 kJ/mol
CH3OH��g��+H2O��g�� ��H2= ��58 kJ/mol
��CO2��g��+H2��g��![]() CO��g��+H2O��g�� ��H3
CO��g��+H2O��g�� ��H3
��֪��Ӧ���е���صĻ�ѧ�������������£�
��ѧ�� | H��H | C��O | C ��CO�еĻ�ѧ���� | H��O | C��H |
E/��kJ/mol�� | 436 | 343 | 1076 | 465 | 413 |
�ش��������⣺
����H3=_____kJ/mol
��25����101 kPa�����£����16g�״���ȫȼ���ͷų�Q kJ����������д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ_______________
��2��25������a mol��L��1��ˮ��b mol��L��1����������Ϻ���Һ�����ԣ����ʱ��Һ��c��NH4+��__________c��Cl����������������������������ú�a��b�Ĵ���ʽ��ʾ���¶���NH3��H2O�ĵ���ƽ�ⳣ��Kb = _________
��3��800��ʱ����2L�ܱ������з�����Ӧ2NO��g����O2��g��![]() 2NO2��g�����ڷ�Ӧ��ϵ�У�n��NO����ʱ��ı仯���±���ʾ��
2NO2��g�����ڷ�Ӧ��ϵ�У�n��NO����ʱ��ı仯���±���ʾ��
ʱ����s�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
����ͼ�б�ʾNO2�仯��������___________��O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v��____

����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����_______
a��v��NO2��=2v��O2��
b��������ѹǿ���ֲ���
c��v����NO����2v����O2��
d�������ڵ��ܶȱ��ֲ���
���𰸡���1����+41
��CH3OH��l��+3/2 O2��g��=CO2+2H2O ��H=��2QkJ/mol
��2�� =��1���� �� b��10��7/��a��b��
��3������b ��1������ 1.5��10��3mol/��L��s�� ��1���� ��2��bc ��1����
��������
�����������1������Ӧ��=��Ӧ���ܼ���-�������ܼ��ܣ�����H1=1076kJ��mol-1+2��436kJ��mol-1-��3��413+343+465��kJ��mol-1=-99kJ��mol-1�����ݸ�˹���ɣ���Ӧ��-��Ӧ��=��Ӧ��������H3=��H2-��H1=-58kJ��mol-1-��-99kJ��mol-1��=+41kJ��mol-1��
����25����101kPa�£�16g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����QkJ��32g�״�ȼ�����ɶ�����̼��Һ̬ˮ�ų�����Ϊ2QKJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+![]() O2��g��=CO2��g��+2H2O��l����H=-2QkJmol-1��
O2��g��=CO2��g��+2H2O��l����H=-2QkJmol-1��
��2����a molL-1�İ�ˮ��b molL-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH-��=1��10-7mol/L����Һ��c��NH4+��=c��Cl-��=![]() mol/L����Ϻ�Ӧǰc��NH3H2O��=
mol/L����Ϻ�Ӧǰc��NH3H2O��=![]() mol/L����Ӧ��c��NH3H2O��=��
mol/L����Ӧ��c��NH3H2O��=��![]() -
-![]() ��mol/L����K=
��mol/L����K=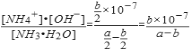 ��
��
��3����NO2�Dz���淴Ӧ����Ũ������ƽ��ʱŨ��ΪNOŨ�ȵı仯����c��NO��=![]() =0.0065mol/L������ͼ�б�ʾNO2�仯��������b��
=0.0065mol/L������ͼ�б�ʾNO2�仯��������b��
��2s����NO��ʾ��ƽ����Ӧ����v��NO��= =3.0��10-3molL-1s-1������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��O2��=
=3.0��10-3molL-1s-1������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��O2��=![]() v��NO��=
v��NO��=![]() ��3.0��10-3molL-1s-1=1.5��10-3molL-1s-1��
��3.0��10-3molL-1s-1=1.5��10-3molL-1s-1��
��a����ʾͬһ����Ӧ���ʣ�v��NO2����ʼ����Ϊv��O2����2��������˵���ﵽƽ�⣬��a����b���淴Ӧ���У���Ӧ��������ܵ����ʵ����ڼ�С��������ѹǿ����ʱ��˵�����������ʵ������ֲ��䣬˵����Ӧ����ƽ�⣬��b��ȷ��c����ͬ���ʱ�ʾ���ʣ�����ƽ��ʱ����������֮�ȵ��ڻ�ѧ������֮�ȣ�V�� ��NO��������O2��=2��1����V�� ��NO��=2v����O2������c��ȷ��d�������������������䣬�����ݻ�Ϊ��ֵ�������ܶ���ʼ���ղ��䣬����˵���ﵽƽ�⣬��d�������ʴ�Ϊbc��

����Ŀ���ο�������a������c����ش����⣺
��a������ֵ��ʹ1g��֬��������Ҫ��KOH�ĺ�������
��b����ֵ��ʹ100g��֬�ӳɵ�Ŀ�����
��c��������֬������ֵ����ֵ�б����£�
������ | �������� | ţ�� | ���� | Ӳ������ | ���� | |
����ֵ | 190 | 180 | 195 | 226 | 193 | 193 |
��ֵ | 90 | 182 | 38 | 38 | 5 | 126 |
��1����������C17H33COO��3C3H5����Է�������884���γɵ��ͣ�����ֵ��
��2���ں����������ʵ��Ĵʾ䣺
�������ͱȻ�����������_________________�࣬���ͱ�ţ�͵�_________________С��Ӳ����
���͵ĵ�ֵС��ԭ����_________ ________��
��3��Ϊʹ��ֵΪ180������100 gӲ������Ҫ��H2�����Ϊ ������״������?
��4���ṹ��ʽΪ ������������ֵΪ430����nΪ
������������ֵΪ430����nΪ
����Ŀ��������������NOx���Ǵ�����Ⱦ��֮һ����ҵ����һ���¶Ⱥʹ�����������NH3��NOx��ԭ����N2��ijͬѧ��ʵ�����ж�NH3��NOx��Ӧ������̽�����ش��������⣺
��1���������Ʊ�

�������ķ���װ�ÿ���ѡ����ͼ�е�_________����Ӧ�Ļ�ѧ����ʽΪ_______________��
��Ԥ�ռ�һƿ����İ�����ѡ����ͼ�е�װ�ã�������˳��Ϊ������װ����______��������������Сд��ĸ��ʾ����
��2����������������ķ�Ӧ
�������ռ�����NH3����ע����X�У�Ӳ�ʲ�����Y�м�����������������NO2�������ü���K1��K2�к�������һ���¶��°�ͼʾװ�ý���ʵ�顣

�������� | ʵ������ | ����ԭ�� |
��K1���ƶ�ע����������ʹX�е����建��ͨ��Y���� | ��Y����_____________ | ����Ӧ�Ļ�ѧ����ʽ ____________ |
��ע���������˻�ԭ�����̶�����װ�ûָ������� | Y����������ˮ�� | ���ɵ���̬ˮ���� |
��K2 | ��_______________ | ��______________ |