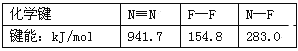��Ŀ����
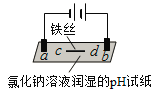
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ�������ͼ��ʾ��

��1�����оٺ�ˮ������һ�ַ���____________��
��2����NaCl��Һ���е�⣬�ڵ����п�ֱ�ӵõ��IJ�Ʒ��H2��________��________��
��3�����������Ѿ����Br2�����������ֽ���õ�Br2��ԭΪBr������Ŀ����____________��
��4����������SO2ˮ��Һ����Br2�������ʿɴ�95%���÷�Ӧ�����ӷ���ʽΪ_____________________________________���ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ�����л�Ӧ�����������__________________________________________________________��
���𰸡�
��1�������������������ӽ��������е�һ��
��2��NaOH Cl2
��3��������Ԫ��
��4��SO2+Br2+2H2O�T4H++2Br-+SO42- ǿ����豸�����ظ�ʴ
��������
�����������1�� �������������������ӽ������Ƚ�����ˮ������
��2����NaCl��Һ���е�⣬�ڵ����������������������������������������ƣ�������ֱ�ӵõ��IJ�Ʒ��H2��NaOH�� Cl2��
��3�����������Ѿ����Br2�����������ֽ���õ�Br2��ԭΪBr������Ŀ����������Ԫ����
��4����������SO2ˮ��Һ����Br2����Ӧ�����ӷ���ʽΪSO2+Br2+2H2O�T4H++2Br-+SO42-����Ӧ�������ᣬ�ڹ�ҵ�����л�Ӧ�����������ǿ����豸�����ظ�ʴ��

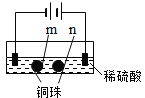
����Ŀ����ʯī�缫������е��ʵ�顣
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a��d����ֽ������b����죬�ֲ���ɫ��c�������Ա仯 | ����ʯī�缫���������ݲ�����n�������ݲ��������� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ���������� ��
A��a��d����2H2O+2e-=H2��+2OH-
B��b����2Cl--2e-=Cl2��
C��c�������˷�Ӧ��Fe-2e-=Fe2+
D������ʵ��һ��ԭ����ʵ�����m��������ͭ
����Ŀ����1���״�����Ҫ�Ļ���ԭ�ϣ��ֿ���ȼ�ϡ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�����Ҫ��Ӧ���£�
��CO��g��+2H2��g��![]() CH3OH��g�� ��H1
CH3OH��g�� ��H1
��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g�� ��H2= ��58 kJ/mol
CH3OH��g��+H2O��g�� ��H2= ��58 kJ/mol
��CO2��g��+H2��g��![]() CO��g��+H2O��g�� ��H3
CO��g��+H2O��g�� ��H3
��֪��Ӧ���е���صĻ�ѧ�������������£�
��ѧ�� | H��H | C��O | C ��CO�еĻ�ѧ���� | H��O | C��H |
E/��kJ/mol�� | 436 | 343 | 1076 | 465 | 413 |
�ش��������⣺
����H3=_____kJ/mol
��25����101 kPa�����£����16g�״���ȫȼ���ͷų�Q kJ����������д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ_______________
��2��25������a mol��L��1��ˮ��b mol��L��1����������Ϻ���Һ�����ԣ����ʱ��Һ��c��NH4+��__________c��Cl����������������������������ú�a��b�Ĵ���ʽ��ʾ���¶���NH3��H2O�ĵ���ƽ�ⳣ��Kb = _________
��3��800��ʱ����2L�ܱ������з�����Ӧ2NO��g����O2��g��![]() 2NO2��g�����ڷ�Ӧ��ϵ�У�n��NO����ʱ��ı仯���±���ʾ��
2NO2��g�����ڷ�Ӧ��ϵ�У�n��NO����ʱ��ı仯���±���ʾ��
ʱ����s�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
����ͼ�б�ʾNO2�仯��������___________��O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v��____
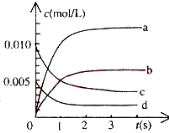
����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����_______
a��v��NO2��=2v��O2��
b��������ѹǿ���ֲ���
c��v����NO����2v����O2��
d�������ڵ��ܶȱ��ֲ���