��Ŀ����
����Ŀ���£�N2H4����Ҫ�������������������ȼ�ϡ�
(1) ��֪ �� 2O2(g)��N2(g) === N2O4(l) ��H��a kJ��mol-1
�� N2(g)��2H2(g) === N2H4(l) ��H��b kJ��mol-1
�� 2H2(g) + O2(g) = 2H2O(g) ��H��c kJ��mol-1
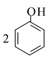
ij�ͺŻ������Һ̬�º�Һ̬N2O4���ƽ�����ȼ��������������Ⱦ�����塣д����Ӧ���Ȼ�ѧ����ʽ____��ƫ�����£�1,1-�����£�Ҳ��һ�ָ���ȼ�ϣ�д����ṹ��ʽ______��
��2���¿��Գ�ȥˮ�е��ܽ��������������ܲ������ѭ����д���÷�Ӧ�Ļ�ѧ����ʽ________�������ϣ�ÿ����64 g�¿ɳ�ȥ��״����O2________L
��3����ѧ��������Ϊȼ�ϵ�ص�ȼ�ϣ���ؽṹ��ͼ1��ʾ��
д����ظ����ĵ缫��Ӧʽ��________��
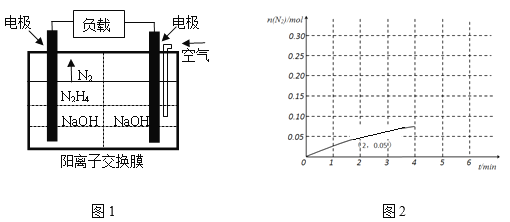
��4��N2H4���ض������£�303K��Pt��Ni�����������Է������ַֽ⣺N2H4(g) ![]() 2H2(g)��N2(g)����2 L���ܱ������м���0.1 mol N2H4(g)�����0-4������N2�����ʵ�����ʱ��ı仯������ͼ2��ʾ��д��0-2������H2��ƽ����Ӧ����v(H2)=________��
2H2(g)��N2(g)����2 L���ܱ������м���0.1 mol N2H4(g)�����0-4������N2�����ʵ�����ʱ��ı仯������ͼ2��ʾ��д��0-2������H2��ƽ����Ӧ����v(H2)=________��
���𰸡�2N2H4(l)��N2O4(l) ===3N2(g)��4H2O(g) ��H��(2c��a��2b) kJ��mol-1  N2H4��O2===2H2O��N2 44.8 N2H4��4OH-��4e-=== N2����4H2O 0.025 mol��L-1��min-1
N2H4��O2===2H2O��N2 44.8 N2H4��4OH-��4e-=== N2����4H2O 0.025 mol��L-1��min-1
��������
��1�����ø�˹�������Һ̬�º�Һ̬N2O4��ȼ��������������Ⱦ��������Ȼ�ѧ����ʽ������ƫ�����µ����֣�1,1-�����£���д�ṹ��ʽ��
��2���¿��Գ�ȥˮ�е��ܽ������������ܲ������ѭ���ĵ������ɴ�д���÷�Ӧ�Ļ�ѧ����ʽ��������ÿ����64 g�¿ɳ�ȥ��״����O2�����
��3������Ϊȼ�ϵ�ȼ�ϵ�أ�ȼ���²��뷴Ӧ�ĵ缫Ϊ��������ʧȥ���ӣ�
��4����ͼ���Կ�����0-2�����ڣ�![]() n(N2)=0.05mol,�ɴ���0-2�����ڵ�v(N2)����������֮�ȵ��ڻ�ѧ������֮�����v(H2)��
n(N2)=0.05mol,�ɴ���0-2�����ڵ�v(N2)����������֮�ȵ��ڻ�ѧ������֮�����v(H2)��
��1���� 2O2(g)��N2(g) === N2O4(l) ��H��a kJ��mol-1
�� N2(g)��2H2(g) === N2H4(l) ��H��b kJ��mol-1
�� 2H2(g) + O2(g) = 2H2O(g) ��H��c kJ��mol-1
���ݸ�˹����2![]() ��
��![]() 2
2![]() ��
��![]() �ٵã�2N2H4(l)��N2O4(l) ===3N2(g)��4H2O(g) ��H��(2c��a��2b) kJ��mol-1��1,1-�����µĽṹ��ʽΪ��
�ٵã�2N2H4(l)��N2O4(l) ===3N2(g)��4H2O(g) ��H��(2c��a��2b) kJ��mol-1��1,1-�����µĽṹ��ʽΪ��![]() ��
��
�������2N2H4(l)��N2O4(l) ===3N2(g)��4H2O(g) ��H��(2c��a��2b) kJ��mol-1��![]() ��
��
��2���������֪���º�������Ӧ�����ɵ�����ˮ���仯ѧ����ʽΪ��N2H4��O2===2H2O��N2��������64 g�¿ɳ�ȥ��״����O2���ΪVL��������������ʽ�ɵã�32:22.4=6.4��V�����V=44.8L��
�������N2H4��O2===2H2O��N2��44.8��
��3������Ϊȼ�ϵ�ȼ�ϵ�أ�ȼ���²��뷴Ӧ�ĵ缫Ϊ��������ʧȥ���ӣ��ڼ��������£��缫��ӦʽΪ��N2H4��4OH-��4e-=== N2����4H2O��
�������N2H4��4OH-��4e-=== N2����4H2O��
��4����ͼ���Կ�����0-2�����ڣ�![]() n(N2)=0.05mol��
n(N2)=0.05mol��![]() c(N2)=0.05mol/2L=0.025mol/L��v��N2��=
c(N2)=0.05mol/2L=0.025mol/L��v��N2��=![]() c/
c/![]() t=
t=![]() =0.0125mol��L-1��min-1����������֮�ȵ��ڻ�ѧ������֮�ȿɵã�v(H2)=2 v��N2��=0.025 mol��L-1��min-1��
=0.0125mol��L-1��min-1����������֮�ȵ��ڻ�ѧ������֮�ȿɵã�v(H2)=2 v��N2��=0.025 mol��L-1��min-1��
�������0.025 mol��L-1��min-1��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����
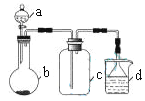
ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ��ˮ | CaO | NH3 | H2O |
B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
C | ϡ���� | Cu | NO2 | H2O |
D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |