��Ŀ����
18��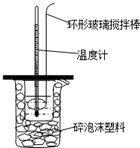 ��50mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1���ձ�����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
��2�����β���������ܷ��û������ʽ�������棿���ܣ���ܡ����ܡ�������ԭ�������ʽ�����״��ȣ�ɢ����������ʹ������¶�ƫ�ͣ������к��ȵIJⶨֵ������ֵƫ�ͣ�
��3��ʵ����������60mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ�������ʵ���������ȷ���������к�����ȣ����ȡ�����ȡ�����
��4����֪��ϡ��Һ�У�ǿ����ǿ����кͷ�Ӧ����1molH2O���ų�57.3kJ����������������Ӧ���Ȼ�ѧ����ʽΪHCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3 kJ/mol
��5��ʵ����������60mL0.50mol/L�Ĵ�����50mL0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������ƫ�ͣ��ƫ�͡���ƫ�ߡ�����ԭ���Ǵ�����������ʣ�������ʵ������������һ����������
���� ��1�������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��2�����ݽ��������ȵ������壬�״��ȣ�
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��4����������кͷ�Ӧ�Ķ���Ҫ����Ȼ�ѧ����ʽ����дԭ��
��5������������ʵ������ȷ�����
��� �⣺��1���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��2�����ʽ�����ĵ����Ժã��״��ȣ�ɢ����������ʹ������¶�ƫ�ͣ������к��ȵIJⶨֵ������ֵƫ�ͣ�
�ʴ�Ϊ�����ܣ����ʽ�����״��ȣ�ɢ����������ʹ������¶�ƫ�ͣ������к��ȵIJⶨֵ������ֵƫ�ͣ�
��3����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ�
�ʴ𰸣�����ȣ���ȣ�
��4����ϡ��Һ�У�ǿ���ǿ����кͷ�Ӧ����1mol H2Oʱ���ų�57.3kJ����������Ӧ���Ȼ�ѧ����ʽ��Ϊ��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3 kJ/mol��
��5��������������ʣ�������ʵ������������һ�������������Ը���60mL0.50mol/L�Ĵ�����50mL0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�������������ʣ�������ʵ������������һ����������
���� ���⿼���й��к��ȵIJⶨ�������к��ȵIJⶨԭ���Լ��������ǽ���Ĺؼ����ѶȲ���

| A�� | 800mL0.5mol/L��NaCl��Һ | B�� | 100mL0.3mol/L��AlCl3��Һ | ||
| C�� | 500mL0.3mol/L��CaCl2��Һ | D�� | 300mL0.3mol/L��MgCl2��Һ |
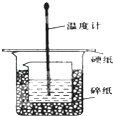 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺��1����ʵ���л�ȱ��һ���������������ǻ��β�����������ڴ�С�ձ��������ĭ���ϵ������DZ��¸��ȣ���ֹ����ɢʧ��
��2����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼��ԭʼ���ݣ���������
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ȣ�2�������Hƫ���ƫ����ƫС�����䡱����
��4�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����������¶ȼƲⶨNaOH��Һ�¶ȵIJ��裬���˲������裬���õ��к��ȡ�H��ƫ���ƫ����ƫС�����䡱����
| A�� |  ��ʾ��������������ʱ��2SO2 ��g��+02 ��g��?2S03 ��g��ת����ϵ�У��������ʾ02��ת���� | |
| B�� |  ��ʾ��0.1mol/L NaOH��Һ�ֱ�ζ���Ũ�ȡ������������ʹ��ᣬ����ʵ��Ϊ�ζ���������� | |
| C�� | 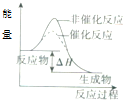 ��ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯 | |
| D�� | 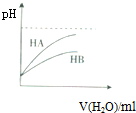 ��ʾ�����£�ϡ��HA��HB�������ϡ��Һʱ����ҺpH���ˮ���ı仯��������HA��HB |
| A�� | ZnΪ������̼Ϊ���� | |
| B�� | ������ӦΪ2NH4++2e-�T2NH3��+H2�� | |
| C�� | ����ʱ������̼�������·����п�� | |
| D�� | ��ʱ������ʹ��ʱ����װ�ĺ�״�����������ʴ���� |
| A�� | Fe�ۣ���ۣ� | B�� | Na2CO3��ĩ��NaHCO3�� | ||
| C�� | NaCl���⣩ | D�� | KMnO4��MnO2�� |
| A�� | CuSO4 •5H2O�����������ﶼ�Ǵ����� | |
| B�� | һ�����ʲ��ǵ���ʾ��Ƿǵ���� | |
| C�� | ϡ���ᡢNaCl��Һ��ʵ���ҳ����ĵ���� | |
| D�� | ��������ɹ㷺����ʳƷ������ |
| Al��OH��3 | Ga��OH��3 | |
| ��ʽ���볣��Ka | 2��10-11 | 1��10-7 |
| ��ʽ���볣��Kb | 1.3��10-33 | 1.4��10-34 |
�������ռ���Һ��Ӧ�����ӷ���ʽ��2Ga+2H2O+2OH-=2GaO2-+3H2����
����X��Һ�л���ͨ��CO2����������������������Al��OH��3��