��Ŀ����
����Ŀ������̪��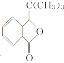 ����һ��������Ѫ�ܼ�����ҩ�����һ�ֺϳ�·����ͼ��
����һ��������Ѫ�ܼ�����ҩ�����һ�ֺϳ�·����ͼ��
��֪��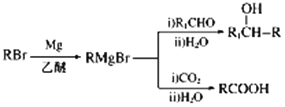
��1��Y��������_____��
��2��H�Ľṹ��ʽΪ___��
��3��X��Y�ķ�Ӧ������_____��H�������ӵ���һ��������ָ_____��
��4��д��W��X�Ļ�ѧ����ʽ��_____��
��5��W�Ķ��������___�֣�����һ���ں˴Ź�����������3���ĽṹΪ___(��ṹ��ʽ)��
��6����ijϩ��Ϊԭ�Ϻϳ�(CH3)3CMgBr�������������̣���ƺϳ�·�ߡ�___
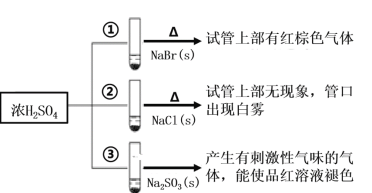
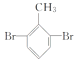
���𰸡�2-�屽��ȩ(�����屽��ȩ)  ������Ӧ Ũ���ᡢ����
������Ӧ Ũ���ᡢ���� 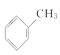 +Br2
+Br2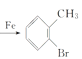 +HBr 10
+HBr 10 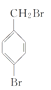 ��
�� ��
��
��������
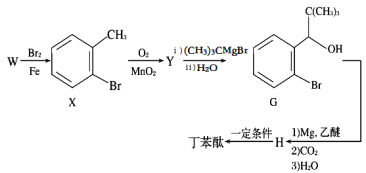
����X�Ľṹ��ʽ��֪��WΪ�ױ�����֪ȩ�����루CH3��3MgBr��H2O��Ӧ����Y�к���ȩ����������Ϊ2-�屽��ȩ(�����屽��ȩ)��G��Mg�����ѷ�Ӧ����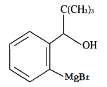 �����������̼��ˮ��Ӧ����H��Ϊ
�����������̼��ˮ��Ӧ����H��Ϊ ���ٷ���������Ӧ���ɶ���̪��
���ٷ���������Ӧ���ɶ���̪��
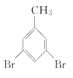
��1��������֪��Y�Ľṹ��ʽΪ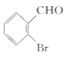 ������Ϊ2-�屽��ȩ(�����屽��ȩ)��
������Ϊ2-�屽��ȩ(�����屽��ȩ)��
��2��������֪��H�Ľṹ��ʽΪ ��
��
��3��X��YΪ����Ϊȩ�����䷴Ӧ������������Ӧ��H�ϳɶ���̪������������Ӧ����������Ũ���ᣬ��������
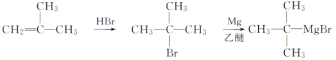
��4��WΪ�ױ������巢��ȡ����Ӧ����X������ʽΪ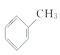 +Br2
+Br2 +HBr��
+HBr��
��5��W(�ױ�)�Ķ��������Է����ࣺ��һ�࣬������ȡ���ڼ��ϣ�ֻ��1�֣��ڶ��࣬һ����ȡ���ڼ��ϣ���һ����ȡ���ڱ����ϣ���3�֣������࣬������ȡ���ڱ����ϣ���6�֣��ϼ���10�֡����У��ں˴Ź�����������3���Ŀ��ܽṹΪ![]() ��
��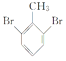 ��
��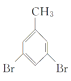 ��
��
��6������֪��Ϣ���(CH3)3CMgBr�Ľṹ��ʽ�����Ƴ���ԭ�ϵ�ϩ��Ϊ2-����ϩ(���춡ϩ)����ϩ�������廯��ӳɣ������������ܼ�����������þ��Ӧ�Ʊ�(CH3)3CMgBr������Ϊ ��
��

����Ŀ��������25 ��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
Ksp��Ka | Ksp=1.8��10��10 | Ksp=9.0��10��12 | Ka=1.8��10��5 | Ka=3.0��10��8 | Ka1=4.1��10��7 Ka2=5.6��10��11 |
A. H2CO3��HCO3����CH3COO����ClO�� ����Һ�п��Դ�������
B. �������Ũ�ȵ�CH3COONa��NaClO������������CH3COONa ��NaClO
C. ��Ũ�Ⱦ�Ϊ1.0��10��3 mol��L��1��KCl��K2CrO4�����Һ�еμ�1.0��10��3 mol��L��1��AgNO3��Һ��CrO42�D���γɳ���
D. ��0.1 mol��L��1 CH3COOH��Һ�еμ�NaOH��Һ����c(CH3COOH):c(CH3COO��)��5��9����ʱ��Һ��pH=5
����Ŀ��POC13�������뵼����Ӽ������άԭ�ϣ�ʵ�����Ʊ�POC13���ⶨ��Ʒ������ʵ��������£�
I.ʵ�����Ʊ�POC13��������������Һ̬PCl3����ȡPOC13��ʵ��װ��(���ȼ��г�������)����ͼ:
���ϣ���Ag��+SCN��=AgSCN�� Ksp(AgCl)>Ksp(AgSCN)��
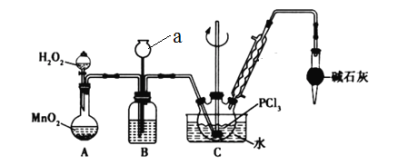
��PCl3��POC13�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | �����ܣ���Ϊ��ɫҺ�壬��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POC13 | 2.0 | 106.0 | 153.5 |
(1)����a������____________________��
(2)B����ʢ���Լ���________������ܵ�������_____________________��
(3)POC13��ˮ��Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)��Ӧ�¶�Ҫ������60~65�棬ԭ���ǣ�____________________________��
II.�ⶨPOC13��Ʒ�ĺ�����ʵ�鲽�裺
���Ʊ�POC13ʵ�����������ƿ�е�Һ����ȴ�����£�ȷ��ȡ29.1g��Ʒ������ʢ��60.00 mL����ˮ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���100.00 mL��Һ��
��ȡ10.00 mL��Һ����ƿ�У�����20.00 mL 3.5mol/L AgNO3����Һ��
�ۼ�����������������ҡ����ʹ�������汻�л��︲�ǡ�
����XΪָʾ������1.00mol/LKSCN��Һ�ζ�����AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥ10.00mLKSCN��Һ��
(5)�������X����ѡ��___________________ ��
(6)����������������������ᵼ�²������______(��ƫ�ߣ�ƫ�ͣ�����Ӱ��)
(7)��Ӧ������POC13�������ٷֺ���Ϊ___________________�� ���ζ��յ㣬��ȡKSCN��Һ���ӿ̶��ߣ����������____________(��ƫ�ߣ�ƫ�ͣ�����Ӱ��)
����Ŀ���״�CH3OH)��һ����Ҫ�Ļ���ԭ�ϣ���ҵ���ж��ַ������Ƶü״���Ʒ
(һ)��CO��H2��CO2�Ʊ��״�
��CO2(g)+H2(g)![]() COg)+H2O(g) H1
COg)+H2O(g) H1
��CO(g)+2H2 (g) ![]() CH3OH(g) ��H2
CH3OH(g) ��H2
��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) H3
CH3OH(g)+H2O(g) H3
��1����֪:��Ӧ�ٵĻ�ѧƽ�ⳣ��K���¶ȵĹ�ϵ���±�
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
������˵����ȷ����______
A.��Ӧ������Ӧ�����ȷ�Ӧ
B.һ��������ܱ������У�ѹǿ���ٱ仯ʱ��˵����Ӧ�ٴﵽƽ��״̬
C.1100��ʱ����Ӧ�ٵ�K����Ϊ1.5
D.��1000��ʱ��[c(CO2)��c(H2)]/[c(CO)��c(H2O)]ԼΪ0.59
��2���Ƚ���H2_____��H3(����>������������������)
��3�������âں͢�������Ӧ�ϳ�CH3OH����֪CO��ʹ��Ӧ�Ĵ��������½�����̼�ȱ�ʾΪf��[n(H2)-n(CO2)]/[n(CO)+n(CO2)]����������f��_____ʱ��ԭ�����������ʸߣ���������ס�������Ը��ڸ�ֵ����̼�ȣ�������_________________________________.
(��)����Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״�:
(i)�Ʊ��ϳ���:CH4(g)+H2Og) ![]() CO(g)+3H2(g) H1>0
CO(g)+3H2(g) H1>0
(ii)�ϳɼ״�:CO(g)+2H2(g) ![]() CH3OH(g) H2>0
CH3OH(g) H2>0
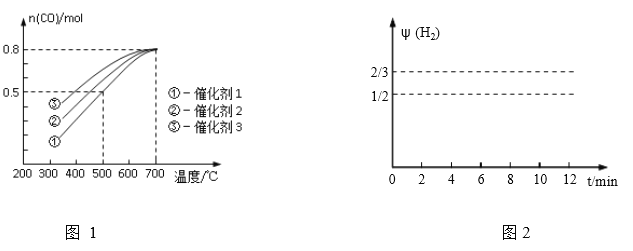
��һ��ѹǿ�£�1 mol CH4(g)��1 mol H2O(g)�����ֲ�ͬ���������·�����Ӧ(i)��������ͬʱ��ʱ��CO�����ʵ���(n)���¶ȱ仯�Ĺ�ϵ��ͼ1
��1������˵����ȷ����_______
A.���ߢ���n(CO)���¶ȱ仯��ԭ��������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�
B.���ִ����У������۵Ĵ�Ч����ã������ܻ����ߵIJ���
C.���¶ȵ���700��ʱ�������ϵĵ���ܶ�û�е���ƽ��
D.���¶ȴ���700��ʱ��CO�����ʵ������ֲ���
��2��500��ʱ����Ӧ(1)�ڴ����ٵ������µ�10mimʱ�ﵽƽ�⣬����ͼ2�л�����Ӧ��1���ڴ�״̬��0��12�����ڷ�Ӧ��ϵ��H2���������![]() (H2)��ʱ��t�仯��������___________________
(H2)��ʱ��t�仯��������___________________
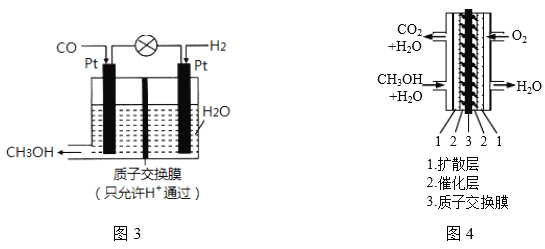
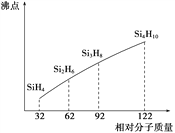
�������о�������COҲ��������������ͨ���绯ѧ�ķ����Ʊ��״���ԭ����ͼ3��ʾ��
��1�������״��ĵ缫��ӦʽΪ___________________��
��2���״�ȼ�ϵ��Ӧ�úܹ㣬�乤��ԭ����ͼ4��д����ع���ʱ�ĸ�����Ӧʽ:___________��