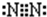��Ŀ����
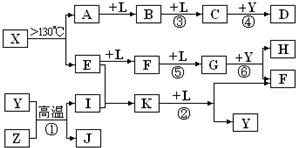
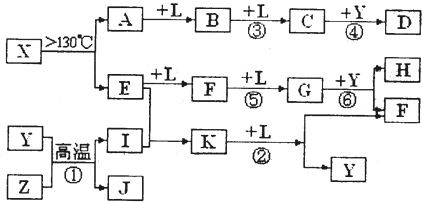
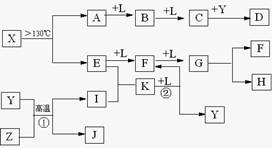
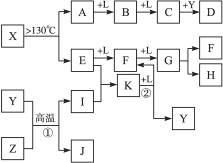
��14�֣���֪A��E��I��L�dz����ķǽ������ʣ�����AΪ����ɫ���壻Z�dz����Ľ������ʣ�B����Է���������A��32��C����Է���������B��16��Y��һ�ֳ�����Һ�壬J�Ǵ����������� D��H��K������Ҫ�Ļ�����Ʒ��X���ӵ����ģ����ͼ��ʾ�����X������Ԫ�ص����ԭ���������18�����п�ͼ�в��ַ�Ӧ��������ȥ��
�Իش��������⣺
��1�������й�X��˵����ȷ���� .
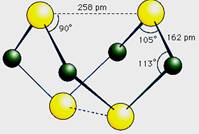
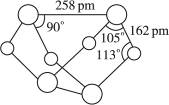
A�������ʵķ���ʽΪS4N4
B�������ʵķ����м��м��Լ����зǼ��Լ�
C�������ʾ��кܸߵ��ۡ��е�
D���������뻯����S2N2��Ϊͬ��������
��2��E�ĵ���ʽΪ ��D�ķ���ʽ ���ڷ�Ӧ�١��ڡ��ۡ��ܡ��ݡ��������ڷ�������ԭ��Ӧ���� ��������ţ�
��3��д����Ӧ�ڵĻ�ѧ����ʽ�� ��
��4��J�����H��ϡ��Һ��Ӧ�����ӷ���ʽΪ ��
1��AB��2�֣���2��N2����ʽ�ԣ�H2SO4���ܣ���2�֣�
��3��4NH3 + 5O2  4NO +6 H2O��3�֣�
4NO +6 H2O��3�֣�
��4��3Fe3O4 + 28H+ + NO3- = 9Fe3+ + NO��+ 14H2O��3�֣�
����