��Ŀ����
��10�֣�����������һ����ɫ����ζ�����Ҳ���ȼ�����壬�ڰ뵼��ӹ���̫���ܵ�غ�Һ����ʾ���������еõ��㷺Ӧ�á�NF3����ͭ�Ĵ���������F2����NH3��Ӧ�õ���
��1����Ԫ�ػ�̬ԭ�ӵļ۵����Ų�ͼΪ ��NF3����ԭ�ӹ�����ӻ�����Ϊ ��
��2��д���Ʊ�NF3�Ļ�ѧ����ʽ�� ��
��3��������HF��NaAlO2��NaCl��6 : 1 : 2�����ʵ���֮��ǡ�÷�Ӧ����HCl��H2O��һ������ˮ����Ҫԭ�ϣ������ʺ�������Ԫ�أ��ڽ�������ұ��������Ҫ���á�������Ϊ���������������� ����λ��Ϊ ���û�������ɫ��Ӧ�������___________ɫ���ܶ�����ζ����Է�����ɫ��Ӧ����ԭ����________________________________________________________��
��1����Ԫ�ػ�̬ԭ�ӵļ۵����Ų�ͼΪ ��NF3����ԭ�ӹ�����ӻ�����Ϊ ��
��2��д���Ʊ�NF3�Ļ�ѧ����ʽ�� ��
��3��������HF��NaAlO2��NaCl��6 : 1 : 2�����ʵ���֮��ǡ�÷�Ӧ����HCl��H2O��һ������ˮ����Ҫԭ�ϣ������ʺ�������Ԫ�أ��ڽ�������ұ��������Ҫ���á�������Ϊ���������������� ����λ��Ϊ ���û�������ɫ��Ӧ�������___________ɫ���ܶ�����ζ����Է�����ɫ��Ӧ����ԭ����________________________________________________________��
��1��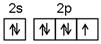 ��2�֣� sp3 (1��)
��2�֣� sp3 (1��)
��2��4NH3+3F2 Cu NF3+3NH4F��2�֣�
��3��Al3+ (1��) 6 ��1�֣� �ƣ�1�֣� ����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ���������ɼ����������ʽ�ͷ���������2�֣�
 ��2�֣� sp3 (1��)
��2�֣� sp3 (1��)��2��4NH3+3F2 Cu NF3+3NH4F��2�֣�
��3��Al3+ (1��) 6 ��1�֣� �ƣ�1�֣� ����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ���������ɼ����������ʽ�ͷ���������2�֣�
��

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ

 ����
����