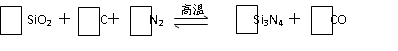��Ŀ����
��9�֣��±��г�ǰ20��Ԫ���е�ijЩԪ�����ʵ�һЩ���ݣ�
�Իش��������⣺
��1����֪HΪNaԪ��,������10��Ԫ���е�һ��������С���ǣ� ����дԪ�ط��ţ�����Ԫ��Bԭ��������10��Ԫ�����̬ԭ�ӵĺ�������Ų�ʽ�� ��
��2��Ԫ��E��C����Ԫ�ؿ��γ�һ����Է�������Ϊ60��һԪ������ӡ���һ�����й��γ� ���Ҽ��� ���м���
��3��������ij����Ԫ��K�ĵ����������ͼ��A����ʾ����KԪ��λ�����ڱ��ĵ� �塣

ͼB���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ������c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ�
�ڲ�ͬͬѧ��ij����Ԫ�ص��⻯��ķе�ı仯���ƻ������������ߡ�����a������b������Ϊ��ȷ���� �������� ��
| Ԫ�� ���� | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶��10-10m�� | 1��02 | 2��27 | 0��74 | 1��43 | 0��77 | 1��10 | 0��99 | 1��86 | 0��75 | 1��17 |
| ���̬ | +6 | +1 | �� | +3 | +4 | +5 | +7 | +1 | +5 | +4 |
| ��ͼ�̬ | -2 | �� | -2 | �� | -4 | -3 | -1 | �� | -3 | -4 |
��1����֪HΪNaԪ��,������10��Ԫ���е�һ��������С���ǣ� ����дԪ�ط��ţ�����Ԫ��Bԭ��������10��Ԫ�����̬ԭ�ӵĺ�������Ų�ʽ�� ��
��2��Ԫ��E��C����Ԫ�ؿ��γ�һ����Է�������Ϊ60��һԪ������ӡ���һ�����й��γ� ���Ҽ��� ���м���
��3��������ij����Ԫ��K�ĵ����������ͼ��A����ʾ����KԪ��λ�����ڱ��ĵ� �塣

ͼB���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ������c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ�
�ڲ�ͬͬѧ��ij����Ԫ�ص��⻯��ķе�ı仯���ƻ������������ߡ�����a������b������Ϊ��ȷ���� �������� ��
(9��)��1��K (1��) 1s22s22P63s23P63d104s1��[Ar] 3d104s1 (2��) ��2�� 7 1 ����1�֣� ��3�� IIIA ��1�֣� IVA��1�֣���
��4��b (1��) A����ʾ���⻯����ˮ����е�������Ԫ�ص��⻯���������������ˮ���Ӽ�������������ǿ��Զ���ڷ��Ӽ�����������1�֣�
��4��b (1��) A����ʾ���⻯����ˮ����е�������Ԫ�ص��⻯���������������ˮ���Ӽ�������������ǿ��Զ���ڷ��Ӽ�����������1�֣�
��

��ϰ��ϵ�д�
�����Ŀ



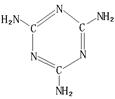
 �D��B���γ����ӻ�����侧���ṹ����ͼ��ʾ����ش��������⡣
�D��B���γ����ӻ�����侧���ṹ����ͼ��ʾ����ش��������⡣

 ���մɲ��ϵ�һ�ֺϳɷ������£�W���Ȼ�����Q���⻯����ȷ�Ӧ�����ɻ�����W(QH2)4��HCL���壻W(QH2)4�ڸ����·ֽ�����Q���⻯����մɲ��ϡ�������ط�Ӧ�Ļ�ѧ����ʽ���������û�ѧʽ��ʾ���� ________
���մɲ��ϵ�һ�ֺϳɷ������£�W���Ȼ�����Q���⻯����ȷ�Ӧ�����ɻ�����W(QH2)4��HCL���壻W(QH2)4�ڸ����·ֽ�����Q���⻯����մɲ��ϡ�������ط�Ӧ�Ļ�ѧ����ʽ���������û�ѧʽ��ʾ���� ________