��Ŀ����
����Ŀ��������һ�������Դ,��������ȡ�봢��������Դ����������о��ȵ㡣
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ������֪:
CH4(g)+H2O(g) = CO(g)+3H2(g)��H=+206.2 kJ/mol
CH4(g)+CO2(g) = 2CO(g)+2H2(g)��H=+247.4 kJ/mol
CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ________________________��
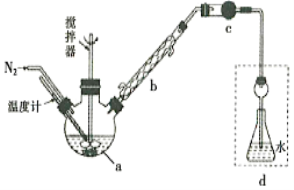
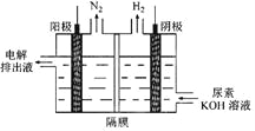
��2���������[CO(NH2)2]�ļ�����Һ�����װ��ʾ��ͼ��ͼ(�����и�Ĥ����ֹ����ͨ��,����������Ϊ���Ե缫)�����ʱ,�����ĵ缫��ӦʽΪ________________________��
��3�� Mg2Cu��һ�ִ���Ͻ�350��ʱ,Mg2Cu��H2��Ӧ,����MgCu2�ͽ���һ�ֽ���Ԫ�ص��⻯��(���������������Ϊ0.077)��Mg2Cu��H2��Ӧ�Ļ�ѧ����ʽΪ_______________��
���𰸡�CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol CO(NH2)2+8OH--6e- = CO32-+N2��+6H2O 2Mg2Cu+3H2![]() MgCu2+3MgH2
MgCu2+3MgH2
��������
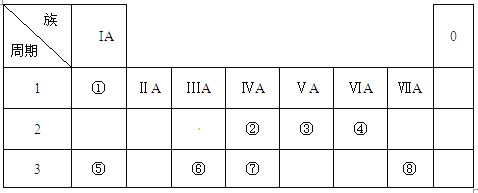
��1�����ø�˹���ɽ�����Ӵ���Ӧ����������Ӧ���������������Ӧ�е�λ����ͨ����Ӽ��ɵ���
��2���������ų�Һ�к��д�����̼���γɷݣ����������������뷴Ӧ��NԪ�صĻ��ϼ����ߣ��Դ�����д�缫��Ӧ�������ܷ�Ӧ������������KOH�Ĺ�ϵ�����
��3��������⻯��ΪRHx������R�����ԭ������Ϊa����![]() =0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2��
=0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2��
��1����֪��CH4(g)+H2O(g) = CO(g)+3H2(g)��H=+206.2 kJ/mol
��CH4(g)+CO2(g) = 2CO(g)+2H2(g)��H=+247.4 kJ/mol
�ݸ�˹��������![]() 2-������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
2-������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
��ˣ�������ȷ������CH4(g)+2H2O(g) = CO2(g) +4H2(g)��H=+165.0 kJ/mol��
��2���������ų�Һ�к��д�����̼���γɷݣ��������������뷴Ӧ����������ӦʽΪCO(NH2)2+8OH--6e- = CO32-+N2��+6H2O��
��ˣ�������ȷ������CO(NH2)2+8OH--6e- = CO32-+N2��+6H2O��
��3��������⻯��ΪRHx������R�����ԭ������Ϊa����![]() =0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2
=0.077����923x=77a��XΪ�����Ļ��ϼۣ����ۿɵ�x=2��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2![]() MgCu2+3MgH2��
MgCu2+3MgH2��
��ˣ�������ȷ������2Mg2Cu+3H2![]() MgCu2+3MgH2��
MgCu2+3MgH2��

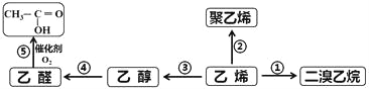
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�