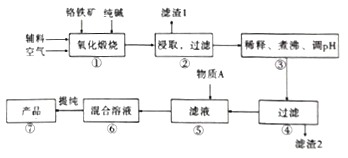
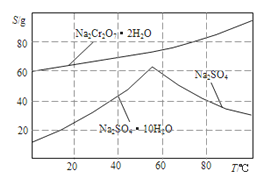
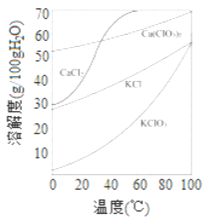
��Ŀ����
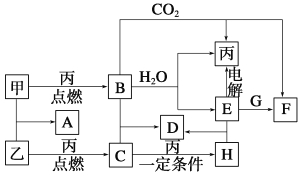
����Ŀ���ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
��ش��������⣺
��1���û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
��2��A�ĵ���ʽΪ________________________________��
��3�����E��ˮ��Һʱ��E��������_______________________
��4��д��B��C�D��D�Ļ�ѧ����ʽ______________________________
д��E��G�D��F�����ӷ���ʽ___________________________________
���𰸡���1��O2��SO3��
��2��![]() ��
��
��3����ǿ��Һ�ĵ���������
��4��Na2O2��SO2===Na2SO4 ��2OH����CO2===CO32��+H2O��
��������
���������B��G����ɫ��Ӧ��Ϊ��ɫ�����B��G�к���NaԪ�أ�C��ʹƷ����Һ��ɫ���Ƴ�CΪSO2���ҡ���Ϊ���ʣ�����ΪS����ΪO2����HΪSO3����Ϊ���ʣ�B�к���NaԪ�أ��˷�Ӧ���ڻ��Ϸ�Ӧ�����ΪNa��AΪNa2S��BΪNa2O2������������ˮ��Ӧ����������NaOH����EΪNaOH����CO2��Ӧ����������Na2CO3����FΪNa2CO3��GΪCO2��DΪNa2SO4����1�����������ƶϣ���ΪO2��HΪSO3��
��2��AΪNa2S���������ӻ�����������Ϊ��![]() ��
��
��3��ˮ��������ʣ�NaOH��ǿ�������ǿ��Һ�ĵ���������
��4��Na2O2��SO2=Na2SO4��2OH����CO2=CO32����H2O��