��Ŀ����
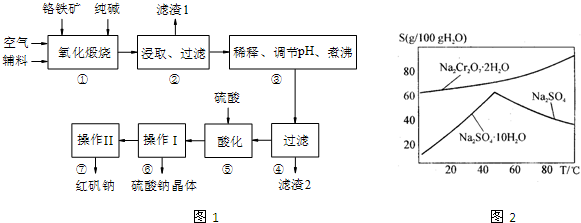
�ظ����ƣ�Na2Cr2O7���㷺������������������ȣ��Ը���ʯ����Ҫ�ɷ�ΪCr2O3��������FeO��A12O3��SiO2�����ʣ�Ϊԭ����ȡ�ظ����Ƶ�������ͼ1��

��1��Cr2O3�ڸ��±���ʱ��Ӧ�Ļ�ѧ����ʽΪ
��2�����������У�����pH��Ŀ����ʹSiO32-��AlO2-�ֱ�ת��Ϊ
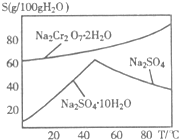
��3��ͼ2��Na2Cr2O7?2H2O��Na2SO4���ܽ�����ߣ�
�����ᴿ�����У�ȥ������Na2SO4��ʵ�鷽������Na2Cr2O7��Na2SO4�����Һ����Ũ����Ȼ��
��4��ij�������Ľ����գ�����ԭ����ͼ3��ʾ��װ�ã��缫Ϊʯī����ͨ����������������Һ�����ԣ�ʵ��Na2CrO4ת��ΪNa2Cr2O7��д�������ĵ缫����ʽ
��5�����ݹ��ұ�����CrO42-�ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5.0��10-7mol?L-1���²����ŷţ�������ˮ�ij��÷���Ϊ�����ˮ�м�������Ա�������BaCrO4�������ټ���ij���Լ����������Ba2+��[��֪��Ksp��BaCrO4��=1.2��10-10]
�ٴ����ʷ���ĽǶȿ�����ȥ�����Ba2+���ӣ����ӵ��Լ�����Ϊ
�ڼ�������Ա��κ�ˮ��Ba2+��Ũ��Ӧ��С��

��1��Cr2O3�ڸ��±���ʱ��Ӧ�Ļ�ѧ����ʽΪ
2Cr2O3+4Na2CO3+3O2
4Na2CrO4+4CO2
| ||
2Cr2O3+4Na2CO3+3O2
4Na2CrO4+4CO2
��
| ||
��2�����������У�����pH��Ŀ����ʹSiO32-��AlO2-�ֱ�ת��Ϊ
H2SiO3
H2SiO3
��Al��OH��3
Al��OH��3
����д��ѧʽ������3��ͼ2��Na2Cr2O7?2H2O��Na2SO4���ܽ�����ߣ�
�����ᴿ�����У�ȥ������Na2SO4��ʵ�鷽������Na2Cr2O7��Na2SO4�����Һ����Ũ����Ȼ��
���ȹ���
���ȹ���
���ɵõ�Na2SO4����ͽϴ�����Na2Cr2O7��Һ����4��ij�������Ľ����գ�����ԭ����ͼ3��ʾ��װ�ã��缫Ϊʯī����ͨ����������������Һ�����ԣ�ʵ��Na2CrO4ת��ΪNa2Cr2O7��д�������ĵ缫����ʽ
2H2O-4e-=O2+4H+
2H2O-4e-=O2+4H+
����5�����ݹ��ұ�����CrO42-�ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5.0��10-7mol?L-1���²����ŷţ�������ˮ�ij��÷���Ϊ�����ˮ�м�������Ա�������BaCrO4�������ټ���ij���Լ����������Ba2+��[��֪��Ksp��BaCrO4��=1.2��10-10]
�ٴ����ʷ���ĽǶȿ�����ȥ�����Ba2+���ӣ����ӵ��Լ�����Ϊ
������
������
���ڼ�������Ա��κ�ˮ��Ba2+��Ũ��Ӧ��С��
2.4��10-4mol?L-1
2.4��10-4mol?L-1
����������1����ͼʾ��֪Cr2O3�ڸ��±���ʱ����Na2CrO4������������ԭ��Ӧ��
��2��SiO32-��AlO2-�����ᷴӦ����H2SiO3��Al��OH��3������
��3���¶Ƚϸ�ʱNa2SO4�ܽ�Ƚ�С��Na2Cr2O7�ܽ�Ƚϴ�Ӧ���ȹ��ˣ�
��4��ͨ����������������Һ�����ԣ�˵��������������4H+��OH-�ŵ磻
��5���ٸ���BaSO4������ˮ������ѡ���Լ���
�ڸ����ܶȻ��������㣮
��2��SiO32-��AlO2-�����ᷴӦ����H2SiO3��Al��OH��3������
��3���¶Ƚϸ�ʱNa2SO4�ܽ�Ƚ�С��Na2Cr2O7�ܽ�Ƚϴ�Ӧ���ȹ��ˣ�
��4��ͨ����������������Һ�����ԣ�˵��������������4H+��OH-�ŵ磻
��5���ٸ���BaSO4������ˮ������ѡ���Լ���
�ڸ����ܶȻ��������㣮
����⣺��1����ͼʾ��֪Cr2O3�ڸ��±���ʱ����Na2CrO4������������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ2Cr2O3+4Na2CO3+3O2
4Na2CrO4+4CO2��
�ʴ�Ϊ��2Cr2O3+4Na2CO3+3O2
4Na2CrO4+4CO2��
��2���ʴ�Ϊ��������������ˮ�����������ա�ˮ��֮���ȥ���ɵ�������������Ӧ������Na2SiO3��NaAlO2�����ʣ�����pH��4.7��������H2SiO3��Al��OH��3�������˳�ȥ��
�ʴ�Ϊ��H2SiO3��Al��OH��3��
��3���¶Ƚϸ�ʱNa2SO4�ܽ�Ƚ�С��Na2Cr2O7�ܽ�Ƚϴ�Ӧ���ȹ��ˣ��ʴ�Ϊ�����ȹ��ˣ�
��4��ͨ����������������Һ�����ԣ�˵��������������4H+���缫��ӦʽΪ2H2O-4e-=O2+4H+��
�ʴ�Ϊ��2H2O-4e-=O2+4H+��
��5���ٸ���BaSO4������ˮ�����ʣ���֪Ӧ���������Σ��ʴ�Ϊ�������Σ�
��5.0��10-7mol?L-1��c��Ba2+��=1.2��10-10��c��Ba2+��=2.4��10-4 mol?L-1��
�ʴ�Ϊ��2.4��10-4 mol?L-1��
| ||
�ʴ�Ϊ��2Cr2O3+4Na2CO3+3O2
| ||
��2���ʴ�Ϊ��������������ˮ�����������ա�ˮ��֮���ȥ���ɵ�������������Ӧ������Na2SiO3��NaAlO2�����ʣ�����pH��4.7��������H2SiO3��Al��OH��3�������˳�ȥ��
�ʴ�Ϊ��H2SiO3��Al��OH��3��
��3���¶Ƚϸ�ʱNa2SO4�ܽ�Ƚ�С��Na2Cr2O7�ܽ�Ƚϴ�Ӧ���ȹ��ˣ��ʴ�Ϊ�����ȹ��ˣ�
��4��ͨ����������������Һ�����ԣ�˵��������������4H+���缫��ӦʽΪ2H2O-4e-=O2+4H+��
�ʴ�Ϊ��2H2O-4e-=O2+4H+��
��5���ٸ���BaSO4������ˮ�����ʣ���֪Ӧ���������Σ��ʴ�Ϊ�������Σ�
��5.0��10-7mol?L-1��c��Ba2+��=1.2��10-10��c��Ba2+��=2.4��10-4 mol?L-1��
�ʴ�Ϊ��2.4��10-4 mol?L-1��
���������⿼���Ϊ�ۺϣ���Ŀ�Ѷ��еȣ�����⣬Ҫ��ְ��������Ϣ���ι��������ʵ����ʣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ҵ���Ը�������Ҫ�ɷ�ΪFeO��Cr2O3��̼���ơ�����������Ϊԭ�������ظ�����Na2Cr2O7?2H20��������Ҫ��ӦΪ��
��4Fe?Cr2O3+8Na2CO3+7O2
8Na2CrO4+2Fe2O3+8CO2
��2Na2CrO4+H2SO4?Na2SO4+Na2Cr2O7+H2O
����˵����ȷ���ǣ�������
��4Fe?Cr2O3+8Na2CO3+7O2
| ||
��2Na2CrO4+H2SO4?Na2SO4+Na2Cr2O7+H2O
����˵����ȷ���ǣ�������
| A����Ӧ�ٺ͢ھ�Ϊ������ԭ��Ӧ |
| B����Ӧ�ٵ���������O2����ԭ����FeO?Cr2O3 |
| C�������£�O2�������Դ���Fe3O3С��NaCrO4�� |
| D������1mol��Na2Cr2O7ʱ������Ӧ���̹�ת��6mol���� |

 �ظ����ƣ�Na2Cr2O7����Ҫ����ӡȾ���Ƹҽҩ����Ƶȣ���ҵ���Ը�������Ҫ�ɷ�FeO?Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7?2H2O������Ҫ��Ӧ���£�
�ظ����ƣ�Na2Cr2O7����Ҫ����ӡȾ���Ƹҽҩ����Ƶȣ���ҵ���Ը�������Ҫ�ɷ�FeO?Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7?2H2O������Ҫ��Ӧ���£�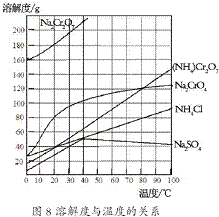 ��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�
��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�