��Ŀ����
 ��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�
��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�����1��������������������ˮ������һ����Ũ�����ữ��ʹ������ת��Ϊ�ظ����ƣ�
����2����������Һ�����ᾧ�������ȹ��ˣ�
����3����������õ��ľ������ܽ⣬�������ᾧ�����ȹ��ˣ�
����4�����������õ�����Һ��ȴ��40�����ҽ��нᾧ����ˮϴ�ӣ�����ظ����ƾ��壮
����5���������ĵõ����ظ����ƺ��Ȼ�藺����ʵ���֮��1��2����������ˮ��������105��110��ʱ�������ַ�Ӧ��
��1������1��һ�����淴Ӧ���÷�Ӧ�����ӷ���ʽΪ
2CrO42-+2H+?Cr2O72-+H2O
2CrO42-+2H+?Cr2O72-+H2O
����2������2��3����ҪĿ����
��ȥ����������
��ȥ����������
����3������4��40�����ҽᾧ������ҪĿ����
����ʹ�����Ʋ�����
����ʹ�����Ʋ�����
����4������5�л�ã�NH4��2Cr2O7���貹��IJ�����
��ȴ�ᾧ�����ˡ�ϴ�Ӽ�����
��ȴ�ᾧ�����ˡ�ϴ�Ӽ�����
����5����NH4��2Cr2O7���ȷֽ���ȡCr2O3�Ļ�ѧ����ʽΪ
��NH4��2Cr2O7�TCr2O3+N2��+4H2O
��NH4��2Cr2O7�TCr2O3+N2��+4H2O
����6����������Ʒ���м���ͺ����ⶨ��
�ټ����Ʒ���Ƿ���K+��������������ж�������
�ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ�����
�ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ�����
����Ϊ�˲ⶨ������Ʒ�У�NH4��2Cr2O7�ĺ�������ȡ��Ʒ0.150g��������ƿ�У���50mLˮ���ټ���2gKI�����������Թ�����ϡ������Һ��ҡ�ȣ���������10min��Ȼ���150mL����ˮ������3mL 0.5%������Һ����0.1000mol/L Na2S2O3����Һ�ζ����յ㣬����Na2S2O3����Һ30.00mL����������Ʒ�У�NH4��2Cr2O7�Ĵ���Ϊ
84%
84%
���ٶ����ʲ��μӷ�Ӧ����֪��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��I2+2S2O2- 3 |
2- 6 |
��������1��������Ϣд��������������������ת��Ϊ�ظ����Ƶķ�Ӧ��
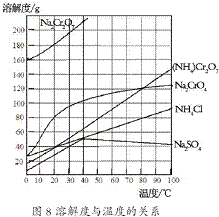
��2������ͼʾ���¶����ߣ������Ƶ��ܽ�Ƚ��ͽ��з�����
��3��������40�����������Ƶ��ܽ����߽��з�����
��4���¶���105��110��ʱ����NH4��2Cr2O7�ܽ��С���ظ�������Һ���ݴ˿���ͨ����ȴ�ᾧ�����ˡ�ϴ�ӣ�������õ��ظ���泥�
��5������������ԭ��Ӧ���ϼ۱仯д����NH4��2Cr2O7���ȷֽ���ȡCr2O3����ʽ��
��6���ٸ�����ɫ��Ӧ��������ӵ�ʵ��������з�����
�ڸ��ݷ�Ӧ�ҳ��ظ��������������ƵĹ�ϵʽ���ȼ�������ĵ���������Ƶ����ʵ������ٸ��ݹ�ϵʽ������ظ���淋����ʵ��������������������Ʒ�У�NH4��2Cr2O7�Ĵ��ȣ�
��2������ͼʾ���¶����ߣ������Ƶ��ܽ�Ƚ��ͽ��з�����
��3��������40�����������Ƶ��ܽ����߽��з�����
��4���¶���105��110��ʱ����NH4��2Cr2O7�ܽ��С���ظ�������Һ���ݴ˿���ͨ����ȴ�ᾧ�����ˡ�ϴ�ӣ�������õ��ظ���泥�
��5������������ԭ��Ӧ���ϼ۱仯д����NH4��2Cr2O7���ȷֽ���ȡCr2O3����ʽ��
��6���ٸ�����ɫ��Ӧ��������ӵ�ʵ��������з�����
�ڸ��ݷ�Ӧ�ҳ��ظ��������������ƵĹ�ϵʽ���ȼ�������ĵ���������Ƶ����ʵ������ٸ��ݹ�ϵʽ������ظ���淋����ʵ��������������������Ʒ�У�NH4��2Cr2O7�Ĵ��ȣ�
����⣺��1������һ������������������ת��Ϊ�ظ����ƣ���ӦΪ���淴Ӧ����Ӧ�ķ���ʽΪ��2CrO42-+2H+?Cr2O72-+H2O��
�ʴ�Ϊ��2CrO42-+2H+?Cr2O72-+H2O��
��2��ͼ8�У��¶����ߣ��������ܽ�Ƚ��ͣ�����ͨ�������¶ȣ�ʹ�������ܽ�Ƚ��Ͷ����������ȹ��˳�ȥ���������ʣ�
�ʴ�Ϊ����ȥ���������ʣ�
��3���������õ�����Һ��ȴ��40�����ң����¶��������Ƶ��ܽ�������Ա��������ƾ���������
�ʴ�Ϊ������ʹ�����Ʋ�������
��4���������ĵõ����ظ����ƺ��Ȼ�藺����ʵ���֮��1��2����������ˮ��������105��110��ʱ�������ַ�Ӧ�������ظ������ܽ�ȴ����ظ���淋��ܽ�ȣ�����ͨ����ȴ�ᾧ�����ˡ�ϴ�Ӻ���õ��ظ���泥�
�ʴ�Ϊ����ȴ�ᾧ�����ˡ�ϴ�Ӽ����
��5���ظ���立�������Cr2O3����Ԫ�ػ��ϼ۽��ͱ���ԭ����Ԫ�ػ��ϼ�һ�������ߣ��������ɵ�������Ӧ�ķ���ʽΪ��NH4��2Cr2O7�TCr2O3+N2��+4H2O��
�ʴ�Ϊ����NH4��2Cr2O7�TCr2O3+N2��+4H2O��
��6����������ɫ��Ӧ��������Һ�еļ������Ƿ���ڣ�����Ϊ���ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ����������ӣ�
�ʴ�Ϊ���ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ�������
����������Ƶ����ʵ���Ϊ��0.1000mol/L��0.03L=0.003mol��
���ݷ�Ӧ��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��I2+2S2O
�T2I-+S4O
��
�ɵù�ϵʽ��Cr2O72-��3I2��6S2O
��n��Cr2O72-��=
n��S2O
��=
��0.003mol=0.0005mol���ظ���淋�����Ϊ��252g/mol��0.0005mol=0.126g��
�ظ���淋���������Ϊ��
��100%=84%��
�ʴ�Ϊ��84%��
�ʴ�Ϊ��2CrO42-+2H+?Cr2O72-+H2O��
��2��ͼ8�У��¶����ߣ��������ܽ�Ƚ��ͣ�����ͨ�������¶ȣ�ʹ�������ܽ�Ƚ��Ͷ����������ȹ��˳�ȥ���������ʣ�
�ʴ�Ϊ����ȥ���������ʣ�
��3���������õ�����Һ��ȴ��40�����ң����¶��������Ƶ��ܽ�������Ա��������ƾ���������
�ʴ�Ϊ������ʹ�����Ʋ�������
��4���������ĵõ����ظ����ƺ��Ȼ�藺����ʵ���֮��1��2����������ˮ��������105��110��ʱ�������ַ�Ӧ�������ظ������ܽ�ȴ����ظ���淋��ܽ�ȣ�����ͨ����ȴ�ᾧ�����ˡ�ϴ�Ӻ���õ��ظ���泥�
�ʴ�Ϊ����ȴ�ᾧ�����ˡ�ϴ�Ӽ����
��5���ظ���立�������Cr2O3����Ԫ�ػ��ϼ۽��ͱ���ԭ����Ԫ�ػ��ϼ�һ�������ߣ��������ɵ�������Ӧ�ķ���ʽΪ��NH4��2Cr2O7�TCr2O3+N2��+4H2O��
�ʴ�Ϊ����NH4��2Cr2O7�TCr2O3+N2��+4H2O��
��6����������ɫ��Ӧ��������Һ�еļ������Ƿ���ڣ�����Ϊ���ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ����������ӣ�
�ʴ�Ϊ���ýྻ�IJ�˿�ھƾ�������������ɫ��Ȼ��պȡ�����������Һ�������ھƾ��ƻ��������գ�ͨ����ɫ�ܲ����۲죬��������ʾ��ɫ˵�����м����ӣ�������
����������Ƶ����ʵ���Ϊ��0.1000mol/L��0.03L=0.003mol��
���ݷ�Ӧ��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��I2+2S2O
2- 3 |
2- 6 |
�ɵù�ϵʽ��Cr2O72-��3I2��6S2O
2- 3 |
| 1 |
| 6 |
2- 3 |
| 1 |
| 6 |
�ظ���淋���������Ϊ��
| 0.126g |
| 0.150g |
�ʴ�Ϊ��84%��
���������⿼�����ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ�ظ���淋ķ���������ؼ��Ƿ��������������������̣���ϵ��ѧ֪ʶ���з����������Ѷ��еȣ�

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ


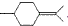 ���������մ��Ľṹ��ʽΪ
���������մ��Ľṹ��ʽΪ
 �ĺϳ�·������ͼ�������Լ����ã��ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�������Լ����ã��ϳ�·������ͼʾ�����£� ��
��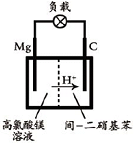 ��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�
��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�