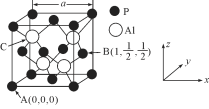
��Ŀ����
����Ŀ������0.1mol��L��1 Na2CO3��Һ������������ǣ� ��
A.0.5 L����Һ�У�Na+�����ʵ���Ũ��Ϊ0.2mol��L��1
B.1 L����Һ�У���CO32������ĿС��0.1NA(NA�ǰ����ӵ�����)
C.��1 L����Һ��ȡ��100 mL����ȡ������Һ��Na2CO3�����ʵ���Ũ��Ϊ0.01mol��L-1
D.ȡ����Һ10 mL����ˮϡ����100 mL��Na2CO3�����ʵ���Ũ��Ϊ0.01mol��L��1
���𰸡�C
��������
A�������ӵ����ʵ���Ũ��Ϊ0.1mol/L��2=0.2mol/L����A��ȷ��
B��Na2CO3��Һ�д���CO32����ˮ�⣬��1 L����Һ�У���CO32������ĿС��0.1NA����B��ȷ��
C����1L����Һ��ȡ��100mL����Һ���о�һ�ԣ���ȡ����Һ��Na2CO3�����ʵ���Ũ��Ϊ0.1mol/L����C����
D��ȡ����Һ10mL����ˮϡ����100mL��Na2CO3�����ʵ���Ũ��Ϊ![]() =0.01mol/L����D��ȷ��
=0.01mol/L����D��ȷ��
�ʴ�ΪC��

��ϰ��ϵ�д�
�����Ŀ