��Ŀ����
����Ŀ����֪��ͭ�����̿����Ҫ�ɷ����±���ʾ��
��ʯ | ��ͭ�� | ���̿� |
��Ҫ�ɷ� | Cu2S��Fe2O3��SiO2 | MnO2��SiO2 |
ij���������������ֿ�ʯΪԭ�ϲ���ʪ��ұ��ͭ���������£�
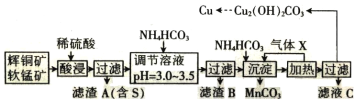
�ش��������⣺
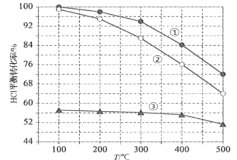
(1)���� X ��Ũ������������̡���д������ X �ĵ���ʽ��______________________________��
(2)��ߡ���������ʵĴ�ʩ��______________________________(������)��
(3)�����ӷ���ʽ��ʾ��������� MnO2 �����ã�______________________________����������Ԫ����______________________________(��Ԫ�ط���)��
(4)��֪ CH3COONH4 ��Һ�����ԣ������£�NH4HCO3 ��Һ pH______________________________(���������<����=��)7��������__________��
(5)����Һ C �п�����ȡһ�ֻ�ѧ���ϣ����Ļ�ѧʽΪ_____________________________��
(6) ����Һ�������ļ�ʽ̼��ͭ���ܴ��нᾧˮ������ͨʽΪ Cu2(OH)2CO3��xH2O��ȷ��ȡa g ��Ʒ�����������أ��Ƶ� CuO �������������������� b g���� x Ϊ______________________________��
���𰸡�![]() �ʵ����������Ũ�ȡ��ʵ������¶ȡ�����ʯ��������Ӵ�������߽��н��裨�������֣�
�ʵ����������Ũ�ȡ��ʵ������¶ȡ�����ʯ��������Ӵ�������߽��н��裨�������֣� ![]() Cu��S ��
Cu��S �� ![]() ��ˮ��̶ȴ���
��ˮ��̶ȴ���![]() (NH4)2SO4
(NH4)2SO4 ![]()
��������
���̷�����֪��ͭ�����̿�������������Cu2S+2MnO2+8H+=2Cu2++S��+2Mn2++4H2O��Fe2O3+6H+=2Fe3++3H2O��SiO2���ܣ����˵õ�����AΪS��SiO2���õ�����Һ������ҺPH��ʹ������ȫ������������BΪ�������������˵õ�����Һ����Ҫ����CuSO4��MnSO4�ȣ�����̼����狀Ͳ�ͨ�백�������˵õ�����ΪMnCO3��������Һ���ȸϳ��������ᾧ�����õ���ʽ̼��ͭ�����˵õ����ٽ���ҺCͨ������Ũ������ȴ�ᾧ����ϴ�Ӹ���õ����ᰱ���壬�ݴ˷�������
(1)�������� X ��Ũ������������̡�������ͼ������Ԫ�صĹ�ϵ��������֪����XӦ��ΪNH3�������ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)��ߡ���������ʵĴ�ʩ���ʵ����������Ũ�ȡ��ʵ������¶ȡ�����ʯ��������Ӵ�������߽��н�����ʵ�֣��ʴ�Ϊ���ʵ����������Ũ�ȡ��ʵ������¶ȡ�����ʯ��������Ӵ�������߽��н��裨�������֣�
(3) ��������ͼ�п�֪�����Cu2S������������������S���ʺ�Cu2+���ʿ�֪MnO2����Cu2S��Ӧ���������ӷ���ʽ![]() ��ʾ���������MnO2�����ã���Ӧ�б�������Ԫ����Cu��S���ʴ�Ϊ��
��ʾ���������MnO2�����ã���Ӧ�б�������Ԫ����Cu��S���ʴ�Ϊ�� ![]() Cu��S��
Cu��S��
(4)��֪CH3COONH4��Һ�����ԣ���˵��CH3COO-��![]() ��ˮ��̶��൱������CH3COOH�����Ա�H2CO3ǿ����
��ˮ��̶��൱������CH3COOH�����Ա�H2CO3ǿ����![]() ��ˮ��̶ȴ���CH3COO-��Ҳ�ʹ���
��ˮ��̶ȴ���CH3COO-��Ҳ�ʹ���![]() ���������£�NH4HCO3��Һ pH��7���ʴ�Ϊ������
���������£�NH4HCO3��Һ pH��7���ʴ�Ϊ������![]() ��ˮ��̶ȴ���
��ˮ��̶ȴ���![]() ��
��
(5)������������ͼ���ѵó�����Һ C��Ҫ����(NH4)2SO4�����������ʣ������Ļ�ѧʽΪ(NH4)2SO4���ʴ�Ϊ��(NH4)2SO4��
(6) ����ȷ��ȡa g ��Ʒ�����������أ��Ƶ� CuO �������������������� b g����CuO ������Ϊ(a-b)g����![]() ���ʸ���ͭԭ���غ��У�
���ʸ���ͭԭ���غ��У�![]() �����Ժ��еĽᾧˮ�����ʵ���Ϊ��
�����Ժ��еĽᾧˮ�����ʵ���Ϊ��![]() �����������غ��У�m(H2O)+m[Cu2(OH)2CO3]=ag������
�����������غ��У�m(H2O)+m[Cu2(OH)2CO3]=ag������![]() ���������ݵã�
���������ݵã�![]() ����ã�x=
����ã�x=![]() �ʴ�Ϊ��
�ʴ�Ϊ��![]() ��
��