��Ŀ����
����Ŀ��д�����и��л���Ľṹ��ʽ��
(1)��0.2 mol��A����������ȫȼ������CO2��H2O��1.2 mol�����ӳɺ�����2,2�������飬��A�Ľṹ��ʽΪ__________________________________��
(2)ijȲ����H2��ּӳ�����2,5-�������飬��Ȳ���Ľṹ��ʽΪ________________________��
(3)ij��̬��100 mL(��״��)�뺬1.43 g�����ˮǡ����ȫ�ӳɣ������ᆳ�ⶨÿ��̼ԭ���϶���һ����ԭ�ӣ������Ľṹ��ʽΪ__________________________________��
(4)ij��1 mol��2 mol HCl��ȫ�ӳɣ����ɵ��ȴ�����������4 mol Cl2��Ӧ��������Ľṹ��ʽΪ____________________________________��
���𰸡�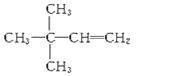
![]() CH2=CH-CH=CH2��
CH2=CH-CH=CH2�� ![]()
��������
���ʷ�Ӧ���������غ㶨�ɣ���Ԫ�����ࡢԭ�Ӹ������䣻Ȳ������C![]() C�������������ӳɷ�Ӧʱ������Cԭ�Ӹ�����2��Hԭ�ӡ�
C�������������ӳɷ�Ӧʱ������Cԭ�Ӹ�����2��Hԭ�ӡ�
(1)��ֻ����C��H��![]() ��0.2 mol��ȼ������CO2��H2O��1.2 mol�����x=6��y=12��A�ɷ����ӳɷ�Ӧ����2,2�������飬��Ϊϩ����˫��ֻ�ܳ�����һ�ˣ���ṹ��ʽΪ��
��0.2 mol��ȼ������CO2��H2O��1.2 mol�����x=6��y=12��A�ɷ����ӳɷ�Ӧ����2,2�������飬��Ϊϩ����˫��ֻ�ܳ�����һ�ˣ���ṹ��ʽΪ�� 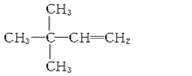 ��(2)2,5-��������Ľṹ��ʽΪCH3-CH��CH3��-CH2-CH2-CH��CH3��- CH3��Ȳ���ӳɺ�����Cԭ�ӳ���2��Hԭ�ӣ���̼���ṹ���䣬����ṹ��ʽ��
��(2)2,5-��������Ľṹ��ʽΪCH3-CH��CH3��-CH2-CH2-CH��CH3��- CH3��Ȳ���ӳɺ�����Cԭ�ӳ���2��Hԭ�ӣ���̼���ṹ���䣬����ṹ��ʽ��![]() ��(3)100mL����µ����壬n=0.1/22.4mol��������������ʵ���Ϊ1molʱ����������ˮ�����ӳɷ�Ӧ�������嵥�ʵ�����Ϊ1.43g
��(3)100mL����µ����壬n=0.1/22.4mol��������������ʵ���Ϊ1molʱ����������ˮ�����ӳɷ�Ӧ�������嵥�ʵ�����Ϊ1.43g ![]() 224��n��Br��=1.43
224��n��Br��=1.43![]() 224/80=4mol����4mol��ԭ�ӣ���Ŀ������ÿ��̼ԭ���϶���һ����ԭ�ӣ������к���4��̼ԭ�ӣ��ɵø����Ľṹ��ʽΪCH2=CH-CH=CH2��(4)ij��1 mol��2 mol HCl��ȫ�ӳɣ�ͬʱ����2molHԭ�ӣ����ɵ��ȴ�����������4 mol Cl2��Ӧ������ȡ����Ӧ��������к���4molHԭ�ӣ���ԭ������2molHԭ�ӣ��Һ���1molC��C��������Ľṹ��ʽ��CH��CH��
224/80=4mol����4mol��ԭ�ӣ���Ŀ������ÿ��̼ԭ���϶���һ����ԭ�ӣ������к���4��̼ԭ�ӣ��ɵø����Ľṹ��ʽΪCH2=CH-CH=CH2��(4)ij��1 mol��2 mol HCl��ȫ�ӳɣ�ͬʱ����2molHԭ�ӣ����ɵ��ȴ�����������4 mol Cl2��Ӧ������ȡ����Ӧ��������к���4molHԭ�ӣ���ԭ������2molHԭ�ӣ��Һ���1molC��C��������Ľṹ��ʽ��CH��CH��