��Ŀ����
17�� ͭ��Ӧ�ý�Ϊ�㷺����ɫ������
ͭ��Ӧ�ý�Ϊ�㷺����ɫ��������1����̬ͭԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��
��2������������Cu2Zn�Ͻ���нϸߵ��۵㡢�ϴ��ǿ�ȡ�Ӳ�Ⱥ���ĥ�ȣ�Cu2Zn�Ͻ�ľ��������ǽ������壮
��3��ij��ͭ����������ӽṹ��1ͼ��ʾ��
�ٸ������д��ڵ���������bc��
a�����Ӽ� b�����ۼ� c����λ��
d����� e�����»���
�ڸ������еڶ����ڵķǽ���Ԫ�صĵ�һ�������ɴ�С��˳����N��O��C��
�۸�������Nԭ�ӵ��ӻ�������sp2��sp3�ӻ���
��4��ͭ�����γɻ�����ľ���ṹ��ͼ2���û�����Ļ�ѧʽΪCu2O��O����λ����8��
���� ��1��Cuԭ�Ӻ��������Ϊ29�������������ԭ������ع���������д��
��2��Cu2Zn�Ͻ�ľ��������ǽ������壻
��3������ͼ��֪��ͭ������N��Oԭ��֮���γ���λ�����ǽ���ԭ��֮���γɹ��ۼ���
�������еڶ�����Ԫ��ΪC��N��O��ͬ����������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�أ�
�������д���C=N˫���е�Nԭ���γ�3���Ҽ�������Nԭ���γ�4���Ҽ�����û�йµ��Ӷԣ�
��4��Cuԭ�Ӵ��ھ����ڲ���Oԭ�Ӵ��ھ������������ģ����þ�̯�����㾧����Cuԭ�ӡ�Oԭ����Ŀ������ȷ������Ļ�ѧʽ�����ϵ�������Oԭ���о�����֮�����Cuԭ����8����Ϊ�������ϲ�4��Cuԭ�ӣ���������ԭ���������⾧�����²�4��Cuԭ�ӣ�
��� �⣺��1��Cuԭ�Ӻ��������Ϊ29�������������ԭ������ع������������������Ų�Ϊ��1s22s22p63s23p63d104s1���ʴ�Ϊ��1s22s22p63s23p63d104s1��
��2��Cu2Zn�Ͻ�ľ��������ǽ������壬�ʴ�Ϊ���������壻
��3������ͼ��֪��ͭ������N��Oԭ��֮���γ���λ�����ǽ���ԭ��֮���γɹ��ۼ�����ѡ��bc��
�������еڶ�����Ԫ��ΪC��N��O��ͬ����������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܣ�N��O��C���ʴ�Ϊ��N��O��C��
�������д���C=N˫���е�Nԭ���γ�3���Ҽ�������Nԭ���γ�4���Ҽ�����û�йµ��Ӷԣ��ֱ��ȡsp2��sp3�ӻ����ʴ�Ϊ��sp2��sp3�ӻ���
��4��Cuԭ�Ӵ��ھ����ڲ���Oԭ�Ӵ��ھ������������ģ�������Cuԭ����ĿΪ8��Oԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ʾ���Ļ�ѧʽΪCu2O�����ϵ�������Oԭ���о�����֮�����Cuԭ��Ϊ�������ϲ�4��Cuԭ�ӣ���������ԭ���������⾧�����²�4��Cuԭ�ӣ�Oԭ����λ��Ϊ8��
�ʴ�Ϊ��Cu2O��8��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����������������ʡ������ܡ���ѧ�����ӻ���ʽ����������ȣ���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� |  ����װ�������� | B�� |  �ռ����� | ||
| C�� |  ��֤���в�����̼̼˫�� | D�� |  ʵ������ȡ���ռ����� |
| A�� | �������������̼���衢�մɺ�̼��ά�ȸ��϶��ɣ�����һ�������л��ϳɲ��� | |
| B�� | ú�к��б������ױ��ȣ�����ͨ��ú�ĸ���õ����������Ƿ��� | |
| C�� | ����һ���Ļ�ѧ�仯�����ԴӺ�ˮ����ȡ�Ȼ��ơ�þ����� | |
| D�� | �ں��������Ƕп�飬�ܼ����ִ��ĸ�ʴ���������������������������� |
| ѡ�� | ���� | ���� |
| A | Al��OH��3��ϡ���� | NaOH��Һ����ˮ |
| B | O2��N2 | H2��Mg |
| C | Cu��NaOH��Һ | FeCl3��Һ��ϡ���� |
| D | SiO2��Cl2 | HF��H2SO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
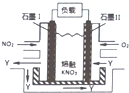 NO2��O2������KNO3��������ȼ�ϵ�أ���ԭ����ͼ���õ����ʹ�ù�����ʯī��缫������������Y��Y��ѭ��ʹ�ã�����˵������ȷ���ǣ�������
NO2��O2������KNO3��������ȼ�ϵ�أ���ԭ����ͼ���õ����ʹ�ù�����ʯī��缫������������Y��Y��ѭ��ʹ�ã�����˵������ȷ���ǣ�������| A�� | NO2��ʯīI��������������Ӧ | |
| B�� | �õ�طŵ�ʱN03-��ʯīI�缫Ǩ�� | |
| C�� | ʯī�������ķ�Ӧ��O2+4e-+2N205=4 N03- | |
| D�� | ��ͬ�����£��ŵ���������ĵ�NO2��O2�������Ϊl��4 |
��1����ij��PM2.5����������ˮ������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
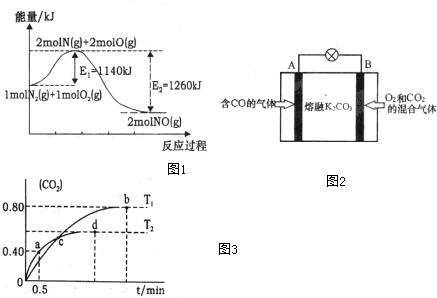
��2��NOx������β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ1���£�
��д��N2��O2��Ӧ���Ȼ�ѧ����ʽN2��g��+O2��g��=2NO��g����H=-120kJ•mol-1��
�ڴ���β��ʱ����װ��ת��װ��ʹNOx��COת��Ϊ����Ⱦ�������ŷţ���д���÷�Ӧ�Ļ�ѧ����ʽ2NOx+2xCO$\frac{\underline{\;����\;}}{\;}$N2+2xCO2
���������뽫CO�����з�Ӧ��ȥ��2CO��g��=2C��s��+O2��g����H��0����������������ܷ�ʵ�֣����ܣ���ǡ����������Ǹ÷�Ӧ���������ؼ��ķ�Ӧ������G=��H-T•��S��G��0��
����������Ϊ����ͼ2��ʾԭ���ԭ��Ҳ���Գ�ȥCO������������ӦʽΪO2+2CO2+4e-=2CO32-�������е�CO32-����A���ƶ����A����B������
��3������I2O5����CO��Ⱦ�ķ�ӦΪ��5CO��g��+I2O5��s��?5CO2��g��+I2��s������ͬ�¶��£���װ������I2O5�����2L�����ܱ�������ͨ��2mol��CO�����CO2�����������ʱ��t�仯������ͼ3����ش��������⣺
��T1ʱ��ѧƽ�ⳣ��K=1024
������˵����ȷ����AD������ĸ��ţ���
A�������������ܶȲ��䣬������Ӧ�ﵽƽ��״̬
B���÷�Ӧ�ġ�H��0
C��d��ʱ������������г���2molCO���ٴ�ƽ���CO2�ĺ�������
D��T1��T2��ѧƽ�ⳣ����С��ϵ��K��T1����K��T2��
| A�� | ���� | B�� | ���� | C�� | ������̼ | D�� | ϡ������ |
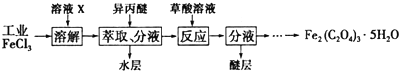
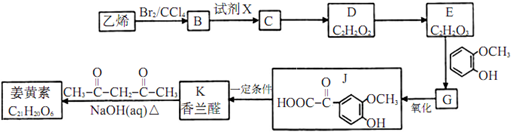

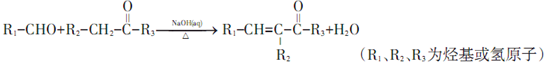
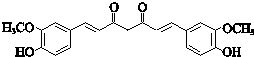 ��
��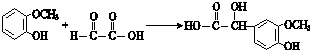 ���䷴Ӧ�����Ǽӳɷ�Ӧ��
���䷴Ӧ�����Ǽӳɷ�Ӧ��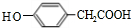 ��д�ṹ��ʽ����
��д�ṹ��ʽ����