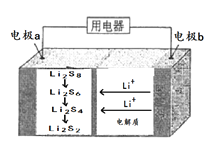
��Ŀ����
����Ŀ��ij����С�����Fe(OH)3������Ʊ�ʵ�鲢���������ʡ�
(1)��������FeCl3��Һ�ֱ�������������У����γɽ������____��
A.��ˮ B.��ˮ C.NaOHŨ��Һ D.NaClŨ��Һ
(2)���мס��ҡ�������ͬѧ����Fe(OH)3������Ʊ�ʵ�飺
�ټ�ͬѧ�IJ�����:ȡһС�ձ�������25 mL����ˮ���������ڣ����ˮ����μ���1~2 mL FeCl3������Һ��������������Һ�ʺ��ɫ��ֹͣ���ȡ������۸ò����Ƿ���ȷ____��
����ͬѧֱ�Ӽ��ȱ���FeCl3��Һ���������Ƿ���ȷ____��
�۱�ͬѧ���ˮ�еμӱ���FeCl3��Һ��Ϊ��ʹ��Ӧ���г�֣����10���ӣ��������Ƿ���ȷ__��
(3)д���Ʊ�Fe(OH)3����Ļ�ѧ����ʽ:_____��֤����Fe(OH)3�������ɵ�ʵ�������________�����õĽ���������___��
(4)Fe(OH)3�����ȶ����ڵ���Ҫԭ����___��
A.����ֱ��С��1 nm B.�����������
C.�����������˶� D.����������ֽ
���𰸡�B ��ȷ ����ȷ ����ȷ FeCl3+3H2O![]() Fe(OH)3(����)+3HCl ��һ���ɼ���ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壻 �����ЧӦ B
Fe(OH)3(����)+3HCl ��һ���ɼ���ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壻 �����ЧӦ B
��������
����ʵ�����Ʊ�������������IJ��������������(1)(2)��
(3)�Ʊ������ԭ��������������ˮ�����������������壻������ж�������ʣ�������������ɢϵ�Ķ������ʣ��ݴ˷������
(4)������к�ǿ��������������������Һ�е����Ӷ�����ɣ���ų⣬���ײ����ϴ����Ӷ��۳����ݴ˷����жϡ�
(1)ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱֹͣ���ȣ��ʴ�Ϊ��B��
(2)���Ʊ�������������ʱ�������ò��������裬��ֹ����۳�������Һ��Ϊ���ɫʱӦ����ֹͣ���ȣ���������Ȼᵼ�½���۳����ʼ�ͬѧ�IJ�����ȷ����ֱ�Ӽ��ȱ���FeCl3��Һ��ٽ��Ȼ���ˮ�⣬�Ҽ��ȴٽ�HCl�ӷ���������Һ���������������Ƶ������������壬����ͬѧ�IJ�������ȷ���ۼ������10���ӣ��ᷢ������ľ۳��������������ʱ�ͬѧ�IJ�������ȷ���ʴ�Ϊ����ȷ������ȷ������ȷ��
(3)�Ʊ������ԭ��������������ˮ�����������������壬��Ӧ�Ļ�ѧ����ʽΪFeCl3+3H2O![]() Fe(OH)3(����)+3HCl��������ж����ЧӦ�����ü��������ʱ������һ�������Ĺ�·��������������ɢϵ�Ķ������ʣ�����һ������ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壬�ʴ�Ϊ��FeCl3+3H2O
Fe(OH)3(����)+3HCl��������ж����ЧӦ�����ü��������ʱ������һ�������Ĺ�·��������������ɢϵ�Ķ������ʣ�����һ������ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壬�ʴ�Ϊ��FeCl3+3H2O![]() Fe(OH)3(����)+3HCl����һ������ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壻�����ЧӦ��
Fe(OH)3(����)+3HCl����һ������ͨ���Ƶõ�Һ�壬�Ӳ���۲쵽һ����������ͨ·����˵���Ƶõ��ǽ��壻�����ЧӦ��
(4)������к�ǿ��������������������Һ�е����Ӷ�����ɣ�����֮����ų⣬���ײ����ϴ������۳����ǽ����ȶ����ڵ���Ҫԭ�ʴ�Ϊ��B��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�����Ŀ���÷�Һ©������ƿ�еμ�Һ�������������ж���ƿ�������Ԥ�����ʵ���������
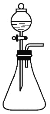
ѡ�� | ��ƿ | ��Һ©�� | Ԥ������ |
A | ����KMnO4��Һ | H2O2��Һ | ��Һ�Ϻ�ɫ����ȥ�Ҳ����������� |
B | Na2S��Һ | ������ˮ | ���ɵ���ɫ���� |
C | Fe(OH)3���� | ���� | �������ɫ���� |
D | Na2CO3��Һ | H2BO3��Һ | ������������ |
A. AB. BC. CD. D