��Ŀ����
Na2SO3�ڿ������ױ����������ʡ�ijͬѧΪ֤��Na2SO3�л�ԭ�ԣ���һƿʵ���ҳ��ڴ�ŵ�Na2SO3������ȡ����������ˮ������һ�������ռ���Һ��������ˮ������Һ��Ϊ��ɫ��
��1���ڼ�����Һ��Br2��Na2SO3��Ӧ�����ӷ���ʽ ��
��2����Ӧ�����Һ����SO32-��SO42-��Br-��OH-�������ӣ��±���ijͬѧ��������SO32-��SO42-��Br-��ʵ�鱨�棬�����δ����IJ��֡�
��ѡ�Լ���2 mol��L-1HCl��1 mol��L-1 H2SO4��l mol��L-1BaCl2��l mol��L-1Ba(NO3)2��1 mol��L-1 KMnO4��CCl4�����Ʊ�����ˮ��Ʒ����Һ��
��3��Ϊ�˲ⶨ������Ʒ�Ĵ��ȣ���ȡ10.0���������250ml��Һ��ȡ��25.00ml������Һ����0.10mol/L������KMnO4��Һ�ζ����յ㡣��Ӧ���ӷ���ʽΪ��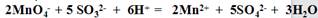
�ظ��������Σ�ÿ������0.10mol/LKMnO4��Һ����ֱ�Ϊ20.02 ml�� 20.00 ml��19.98 ml�������ԭ������Na 23 S 32 O 16)
�ټ�����Ʒ��Na2SO3����������Ϊ �����������3λ��Ч���֣�
�ڲ���ʱ����δ��0.10mol/L������KMnO4��Һ��ϴ�ζ��ܣ��ᵼ�²ⶨ��� �����ƫ�ߡ�����ƫ�͡���û��Ӱ�족��
��1���ڼ�����Һ��Br2��Na2SO3��Ӧ�����ӷ���ʽ ��
��2����Ӧ�����Һ����SO32-��SO42-��Br-��OH-�������ӣ��±���ijͬѧ��������SO32-��SO42-��Br-��ʵ�鱨�棬�����δ����IJ��֡�
��ѡ�Լ���2 mol��L-1HCl��1 mol��L-1 H2SO4��l mol��L-1BaCl2��l mol��L-1Ba(NO3)2��1 mol��L-1 KMnO4��CCl4�����Ʊ�����ˮ��Ʒ����Һ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ�����Թ��У��������2mol��L-1HCl���ٵμ�����1 mol��L-1BaCl2 ��Һ�� | �а�ɫ�������ɣ�֤������Һ�к��С�SO42- �� |
| ����� | | |
| ����� | | |
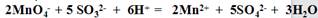
�ظ��������Σ�ÿ������0.10mol/LKMnO4��Һ����ֱ�Ϊ20.02 ml�� 20.00 ml��19.98 ml�������ԭ������Na 23 S 32 O 16)
�ټ�����Ʒ��Na2SO3����������Ϊ �����������3λ��Ч���֣�
�ڲ���ʱ����δ��0.10mol/L������KMnO4��Һ��ϴ�ζ��ܣ��ᵼ�²ⶨ��� �����ƫ�ߡ�����ƫ�͡���û��Ӱ�족��
33����1��SO32- + Br2 + 2 OH- = H2O + SO42- + 2Br- ��3�֣�δ��ƽ��1�֣�
��2����8�֣�
˵���������ֻ�����ᣬ�۲��������ݲ����������֡�
��3���� 0.630��63.0% ��3�֣�δע����Ч���ֿ�1�֣� �� ƫ����2�֣�.
��2����8�֣�
| | ʵ����� | Ԥ����������� |
| ����� | ����1��ȡ������������ϲ���Һ���Թ��У�����������ˮ���۲���Һ����ɫ����2�֣������ټ���CCl4�������ù۲���ɫҲ�ɣ� | ��Һ�ʳȻ�ɫ��֤������Һ�к�Br-����2�֣����²�Һ��ʳȺ�ɫ��֤������Һ�к�Br-���� |
| ����2����ȡ��������Һ���Թ��У�����������2 mol��L-1����ֱ������������Ϊֹ���ټ���������ˮ���۲���Һ����ɫ����2�֣������ټ������Ȼ�̼�������ú�۲���ɫҲ�ɣ� | ��Һ�ʳȻ�ɫ��֤������Һ�к�Br-����2�֣����²�Һ��ʳȺ�ɫ��֤������Һ�к�Br-���� | |
| ����� | ����1��ȡ��������������ϲ���Һ���Թ��У�����2��Ʒ�죬�۲���Һ����ɫ����2�֣� | ��ɫ��ȥ�������SO32-����ɫ����ȥ������SO32-����2�֣� |
| ����2����ȡ��������Һ���Թ��У��������2mol/L���ᣬ�ٵ���2��Ʒ��۲���Һ����ɫ����2�֣� | ��ɫ��ȥ�������SO32-����ɫ����ȥ������SO32-����2�֣� |
˵���������ֻ�����ᣬ�۲��������ݲ����������֡�
��3���� 0.630��63.0% ��3�֣�δע����Ч���ֿ�1�֣� �� ƫ����2�֣�.
�������: ��1���ڼ�����Һ��Br2��Na2SO3����������ԭ��Ӧ�����ȶ������ӣ����������ᣬ�������ӷ���ʽΪ��SO32- + Br2 + 2 OH- = H2O + SO42- + 2Br- ��
��2��ʵ���Ŀ���Ǽ�����Һ�к��е�SO32-��SO42-��Br- �����Կ�����ѡ����Ӧ��ʵ�ʽ��м��顣SO42- ����ѡ��BaCl2 ��Һ������Ӧ��������������İ�ɫ���������֤��SO42- ����Br- ���飬���������䵥�����ױ��л��ܼ���ȡ����ɫ�����������Կ��Լ���ǿ������������ˮ���л��ܼ�CCl4 ������SO32- �н�ǿ��ԭ�ԣ��Һ�ǿ�ᷴӦ�����д̼�����ζ�����壬�ܹ�Ư��Ʒ����Һ�����Կ�������Ư���Խ��м��顣���Ծ��岽�������
| | ʵ����� | Ԥ����������� |
| ����� | ����1��ȡ������������ϲ���Һ���Թ��У�����������ˮ���۲���Һ����ɫ����2�֣������ټ���CCl4�������ù۲���ɫҲ�ɣ� | ��Һ�ʳȻ�ɫ��֤������Һ�к�Br-����2�֣����²�Һ��ʳȺ�ɫ��֤������Һ�к�Br-���� |
| ����2����ȡ��������Һ���Թ��У�����������2 mol��L-1����ֱ������������Ϊֹ���ټ���������ˮ���۲���Һ����ɫ����2�֣������ټ������Ȼ�̼�������ú�۲���ɫҲ�ɣ� | ��Һ�ʳȻ�ɫ��֤������Һ�к�Br-����2�֣����²�Һ��ʳȺ�ɫ��֤������Һ�к�Br-���� | |
| ����� | ����1��ȡ��������������ϲ���Һ���Թ��У�����2��Ʒ�죬�۲���Һ����ɫ����2�֣� | ��ɫ��ȥ�������SO32-����ɫ����ȥ������SO32-����2�֣� |
| ����2����ȡ��������Һ���Թ��У��������2mol/L���ᣬ�ٵ���2��Ʒ��۲���Һ����ɫ����2�֣� | ��ɫ��ȥ�������SO32-����ɫ����ȥ������SO32-����2�֣� |
��3������ʵ�����ݿɵã�ƽ��ÿ�εζ�����KMnO4 ��Һ���V=��20.02 ml+20.00 ml+19.98 ml����3="20.00" ml���ɷ���ʽ��֪�ζ���Ӧ��n(KMnO4 ): n(Na2SO3 )=2:5������n(Na2SO3 )="2.5" n(KMnO4 )="2.5��20.00" ml��10-3��0.10mol/L="0.005" mol��������Ʒ���ܵ�n(Na2SO3 )="10��0.005" mol=0.050mol������Na2SO3 ��Ʒ�Ĵ���=0.050mol��126g/mol��10.0g��100%=63.0%��
�ڸ���c��=c�ꡤV��/V���֪����δ��0.10mol/L������KMnO4��Һ��ϴ�ζ��ܣ����ʹ��ҺKMnO4��Ũ�ȼ�С��Ũ�ȼ�С������ĸ���ı�Һ������V�������´���Һ��Ũ��ƫ�ߡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 Cu +H2O
Cu +H2O Cu
Cu