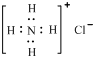��Ŀ����
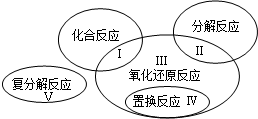
��֪������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ������ͼ��ʾ����������ˮ�μӻ����ɵļ��ַ�Ӧ��
��CaO+H2O =Ca(OH)2
��2Na+H2O=2NaOH+H2��
��H2+CuO Cu +H2O
Cu +H2O
��3S+6NaOH 2Na2S +Na2SO3 +3H2O
2Na2S +Na2SO3 +3H2O
��NaOH+HCl=NaCl+H2O
��ش��������⣺
��1����Ӧ����ˮ ������ĸ����
��2����Ӧ�۱�������Ԫ���� ����Ԫ�ط��ţ���
��3��������Ӧ�У������������� ������ţ���
��4��д��һ�ַ���������Ҽ��г�����������ˮ���ɵ����ӷ���ʽ�� ��

��CaO+H2O =Ca(OH)2
��2Na+H2O=2NaOH+H2��
��H2+CuO
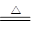 Cu +H2O
Cu +H2O��3S+6NaOH
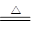 2Na2S +Na2SO3 +3H2O
2Na2S +Na2SO3 +3H2O��NaOH+HCl=NaCl+H2O
��ش��������⣺
��1����Ӧ����ˮ ������ĸ����
| A���������� |
| B���ǻ�ԭ�� |
| C���������������ǻ�ԭ�� |
| D���Ȳ����������ֲ��ǻ�ԭ�� |
��3��������Ӧ�У������������� ������ţ���
��4��д��һ�ַ���������Ҽ��г�����������ˮ���ɵ����ӷ���ʽ�� ��
��1��D ��2��H ��3����
��4��H2CO3+Ca2++2OH-=CaCO3+2H2O��ֻҪ�������⣺���г�����������ˮ���ɵĸ��ֽⷴӦ�����ӷ���ʽ��
��4��H2CO3+Ca2++2OH-=CaCO3+2H2O��ֻҪ�������⣺���г�����������ˮ���ɵĸ��ֽⷴӦ�����ӷ���ʽ��
�����������1��CaO+H2O =Ca(OH)2�÷�Ӧ�У�ˮ������H��OԪ�ػ��ϼ۾�Ϊ�ı䣬��ˮ�Ȳ����������ֲ��ǻ�ԭ����ѡ��Dѡ���2��H2+CuO
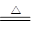 Cu +H2O���ݷ�Ӧ��֪��HԪ�ػ��ϼ����ߣ�CuԪ�ػ��ϼ۽��ͣ��ʱ�������Ԫ��ΪHԪ�ء���3��������Ϊ������ԭ��Ӧ���Ҳ������û������ϡ��ֽⷴӦ���ʢܷ������⡣��4��������Ϊ���ֽⷴӦ�����г�����ˮ���ɡ�H2CO3+Ca2++2OH-=CaCO3+2H2O
Cu +H2O���ݷ�Ӧ��֪��HԪ�ػ��ϼ����ߣ�CuԪ�ػ��ϼ۽��ͣ��ʱ�������Ԫ��ΪHԪ�ء���3��������Ϊ������ԭ��Ӧ���Ҳ������û������ϡ��ֽⷴӦ���ʢܷ������⡣��4��������Ϊ���ֽⷴӦ�����г�����ˮ���ɡ�H2CO3+Ca2++2OH-=CaCO3+2H2O
��ϰ��ϵ�д�
�����Ŀ

 H2SO3 + OH�C �� �� ��д�����ӷ���ʽ��
H2SO3 + OH�C �� �� ��д�����ӷ���ʽ��
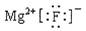
 HSO3 -+H+ K=1.5��10-2
HSO3 -+H+ K=1.5��10-2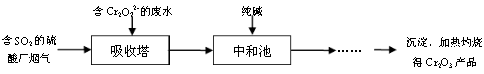
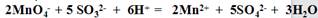
 ���ӵĿռ乹��Ϊ���������ͣ�CO2��C2H2��Ϊֱ���ͷ���
���ӵĿռ乹��Ϊ���������ͣ�CO2��C2H2��Ϊֱ���ͷ��� ��NH4Cl�ĵ���ʽΪ
��NH4Cl�ĵ���ʽΪ