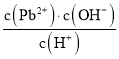��Ŀ����
����Ŀ����(Se)����(Te)�������������ܣ����㷺����ұ�𡢻�����ҽҩ����������ҵ����ͭ������(����Cu��Cu2S��Cu2Se��Cu2Te��)Ϊԭ���Ʊ������ڵ�һ��������������ͼ��ʾ��
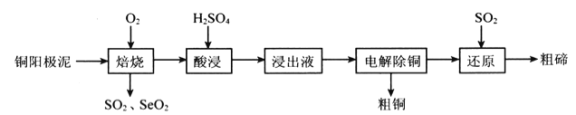
��֪���������������TeO2�����ᷴӦ����TeOSO4��
(1)����ʱͨ������ʹͭ��������ڣ�Ŀ����________________��
(2)SeO2��SO2�Ļ����������ˮ�����Ƶõ���Se���÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ_____________����֪25��ʱ��������(H2SeO3)��Ka1=2.5��10-3��Ka2=2.6��10-7��NaHSeO3��Һ��pH________7(�>������<����=��)��������____________________��
(3)������Һ�������ʳɷֳ���TeOSO4�⣬����_______________���������������п���ѭ��ʹ�õ�������___________________��
(4)����ԭ������������Te�Ļ�ѧ����ʽΪ_______________________��TeҲ����ͨ�����Ի����µ��Na2TeO3��Һ��ã��������ĵ缫��ӦʽΪ___________________��
(5)���������ĺ����������·����ⶨ��
��Se+2H2SO4(Ũ)=2SO2��+SeO2+2H2O��
��SeO2+4KI+4HNO3=Se+2I2+4KNO3+2H2O��
��I2+2Na2S2O3=Na2S4O6+2NaI
ͨ����Na2S2O3����Һ�ζ���Ӧ�������ɵ�I2���������ĺ�����ʵ����ȷ����0��1200 g������Ʒ���ζ�������0.2000 mol��L-1Na2S2O3��Һ24��00 mL���������Ʒ��������������Ϊ_______________��
���𰸡� ����������ͭ������ĽӴ�������ӿ췴Ӧ���� 2:1 < HSeO3-��ˮ�ⳣ��![]() ������Ka1<Ka2����NaHSeO3��Һ��pH<7 CuSO4 H2SO4 2SO2+TeOSO4+3H2O=Te+3H2SO4 TeO32-+3H2O+4e-=Te+6OH- 79%
������Ka1<Ka2����NaHSeO3��Һ��pH<7 CuSO4 H2SO4 2SO2+TeOSO4+3H2O=Te+3H2SO4 TeO32-+3H2O+4e-=Te+6OH- 79%
�����������������(1)���������ʱ�������Ӵ�����Ӵ���
(2)SeO2��SO2�Ļ����������ˮ�����Ƶõ���Se��Se���ϼ۽��ͣ�����SO2����Ԫ�������������ᣬ���ݵ����غ��ж����������뻹ԭ��������ʵ���֮�ȡ�����HSeO3-��ˮ�ⳣ���͵���ƽ�ⳣ����С�Ƚ��ж�NaHSeO3��Һ��pH��
(3)����Ԫ���غ����������Һ�������ʳɷ֡�
(4) ����ԭ��������SO2��TeOSO4����Te��Na2TeO3����Te������ԭ��Ӧ������TeO32-�õ�������Te��
(5)����������ԭ��Ӧ��ϵʽ������������������
������(1) ���������ʱ�������Ӵ�����Ӵ�Ŀ���Ǽӿ췴Ӧ���ʡ�
(2)SeO2��SO2�Ļ����������ˮ�����Ƶõ���Se������ʽΪSeO2+2SO2+2H2O=Se+2H2SO4������������H2SO4����ԭ������Se�����������뻹ԭ��������ʵ���֮��Ϊ2:1��HSeO3-��ˮ�ⳣ��![]() ��HSeO3-�ĵ���ƽ�ⳣ��2.6��10-7���������ˮ�⣬����NaHSeO3��Һ��pH<7��
��HSeO3-�ĵ���ƽ�ⳣ��2.6��10-7���������ˮ�⣬����NaHSeO3��Һ��pH<7��
(3) ����Ԫ���غ㡰����Һ�������ʳɷֳ���TeOSO4�⣬����CuSO4�����������п���ѭ��ʹ�õ�������H2SO4��
(4) ����ԭ��������SO2��TeOSO4����Te�ķ���ʽΪ2SO2+TeOSO4+3H2O=Te+3H2SO4��Na2TeO3����Te������ԭ��Ӧ������TeO32-�õ�������Te��������ӦʽΪTeO32-+3H2O+4e-=Te+6OH- ��
(5)��Se������Ϊxg�����ݹ�ϵʽ
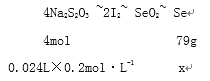
![]()
X=0.0948g
�������Ʒ��������������Ϊ![]() 79% ��
79% ��

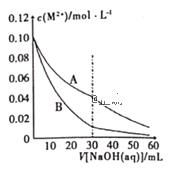
����Ŀ��(1)���������õ������£�NH4+����������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ���£�

�ٵ�һ����Ӧ��________(��������������������)��Ӧ���ж�������________________��
��1molNH4+(aq)ȫ��������NO3-(aq)���Ȼ�ѧ����ʽ��___________________________��
(2)��֪��2CO(g)+O2(g)=2CO2(g)����H=-566kJ��mol-1��
Na2O2(s)+CO2(g)=Na2CO3(s)+0.5O2(g)����H=-226kJ��mol-1��
��CO(g)��Na2O2(s)��Ӧ�ų�509kJ����ʱ������ת����ĿΪ________________��
(3)��֪H2(g)+Br2(l)=2HBr(g)����H=-72kJ��mol-1������1molBr2(l)��Ҫ���յ�����Ϊ30kJ����������������±���
���� | H2(g) | Br2(g) | HBr(g) |
1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����(kJ) | 436 | 200 | a |
�����a=______________��
����Ŀ���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״���![]()
![]()
��1���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��![]()
�¶� | 250�� | 300�� | 350�� |
K |
|
|
|
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ![]() ______________��
______________��![]() __________
__________![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
��ij�¶��£���![]() ��
��![]() ����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���
����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���![]() ����CO��ת����Ϊ____________����ʱ���¶�Ϊ_________��
����CO��ת����Ϊ____________����ʱ���¶�Ϊ_________��
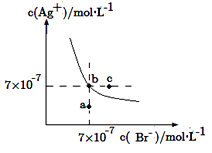
��2����![]() ��
��![]() ��Һ�м���
��Һ�м���![]() �����ᣬ���ɳ�������֪���¶���AgCl��
�����ᣬ���ɳ�������֪���¶���AgCl��![]() ��������Һ������仯������㣺
��������Һ������仯������㣺
����ȫ��������Һ��![]() ________��
________��
����ȫ��������Һ��![]() ________��
________��