��Ŀ����
����Ŀ���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״���![]()
![]()
��1���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��![]()
�¶� | 250�� | 300�� | 350�� |
K |
|
|
|
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ![]() ______________��
______________��![]() __________
__________![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
��ij�¶��£���![]() ��
��![]() ����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���
����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���![]() ����CO��ת����Ϊ____________����ʱ���¶�Ϊ_________��
����CO��ת����Ϊ____________����ʱ���¶�Ϊ_________��
��2����![]() ��
��![]() ��Һ�м���
��Һ�м���![]() �����ᣬ���ɳ�������֪���¶���AgCl��
�����ᣬ���ɳ�������֪���¶���AgCl��![]() ��������Һ������仯������㣺
��������Һ������仯������㣺
����ȫ��������Һ��![]() ________��
________��
����ȫ��������Һ��![]() ________��
________��
���𰸡�![]()
![]()
![]() 250��
250�� ![]()
![]()
��������
��1����.ƽ�ⳣ������������Ũ����֮�����Է�Ӧ��Ũ����֮����![]() ���¶����ߣ�ƽ�ⳣ����С����÷�Ӧ�Ƿ��ȷ�Ӧ��
���¶����ߣ�ƽ�ⳣ����С����÷�Ӧ�Ƿ��ȷ�Ӧ��
�ʴ�Ϊ��![]() ��
��![]() ��
��
��.ƽ��ʱ���![]() ���������ʵ���Ϊ
���������ʵ���Ϊ![]()
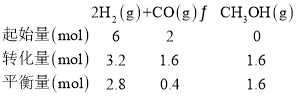
һ����̼��ת����Ϊ��![]() ��ƽ�ⳣ��
��ƽ�ⳣ��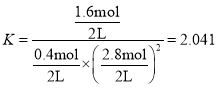 �����ʱ�¶�Ϊ��250����
�����ʱ�¶�Ϊ��250����
�ʴ�Ϊ��80%��250����
��2����.�������������Ӧ�����ʵ���֮����1��1��
![]() ��
��![]() ��Һ�������������ʵ���Ϊ��
��Һ�������������ʵ���Ϊ��![]() ��
��
![]() ����������ʵ���Ϊ��
����������ʵ���Ϊ��![]() ����������ʣ�࣬
����������ʣ�࣬
��Ӧ������ʣ��������ӵ����ʵ���Ϊ��![]() �������ӵ�Ũ��Ϊ��
�������ӵ�Ũ��Ϊ��![]() ��
��
�����ӵ�Ũ��Ϊ��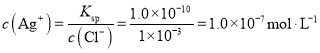 ��
��
�ʴ�Ϊ��![]() ��
��
��.![]() ����������ʵ���Ϊ��
����������ʵ���Ϊ��![]() ��
��![]() ��
��
�����ӵ�Ũ��Ϊ��![]() ��
��![]() ��
��
�ʴ�Ϊ��2��

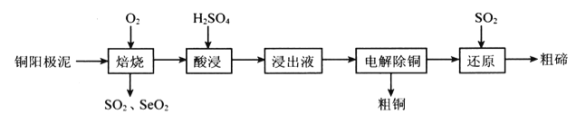
����Ŀ������ͨѶ�ǹ��ά�����źŵ�һ��ͨѶ�ֶΣ��ϳɹ��ά�������裨һ����Ϳ�㣩�Ĺ����������£�

�ش��������⣺
��1����Ӧ��Ļ�ѧ����ʽΪ2C+SiO2 ![]() Si+2CO�������л�ԭ��Ϊ______������Si�����ڱ���λ��______���÷�Ӧ�漰�ĸ���Ӧ������C+SiO2
Si+2CO�������л�ԭ��Ϊ______������Si�����ڱ���λ��______���÷�Ӧ�漰�ĸ���Ӧ������C+SiO2 ![]() Si+CO2����̼���㣩��______��̼��������
Si+CO2����̼���㣩��______��̼��������
��2������Ӧ�����õ����Ȼ����Ʒ���������������£�
������� |
|
|
| HCl |
|
|
�������� |
|
|
|
|
|
|
�е� |
|
|
|
|
|
|
ͼ��������X��������Ϊ______��![]() �ĵ���ʽΪ______��
�ĵ���ʽΪ______��
��3����Ӧ���Ļ�ѧ����ʽΪ3SiCl4+4NH3 ![]() Si3N4+12HCl������2L�����ܱ�������Ͷ��1mol
Si3N4+12HCl������2L�����ܱ�������Ͷ��1mol![]() ��1mol
��1mol![]() ��6min��Ӧ��ȫ����
��6min��Ӧ��ȫ����![]() min�ڣ�HCl��ƽ����Ӧ����Ϊ______molL-lmin-l����Ӧ������������������ͬ����Ӧ��ѧ����ʽΪ______����Ӧ���е�ԭ����
min�ڣ�HCl��ƽ����Ӧ����Ϊ______molL-lmin-l����Ӧ������������������ͬ����Ӧ��ѧ����ʽΪ______����Ӧ���е�ԭ����![]() ��
��![]() �ڼ��������¿ɹ���ȼ�ϵ�أ���������Ӧ�ĵ缫����ʽΪ______��
�ڼ��������¿ɹ���ȼ�ϵ�أ���������Ӧ�ĵ缫����ʽΪ______��