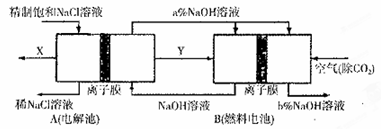
��Ŀ����
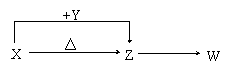
��10�֣�X��Y��Z��W���ֳ������������X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���塣�����ֻ������������ת����ϵ(���ַ�Ӧ����P��Ӧ��������ȥ)��
��ش�
��W�Ļ�ѧʽ��____________________��
��X��Y����Һ�з�Ӧ�����ӷ���ʽ��_______________________________��
�Ǣٽ�4.48 L(������Ϊ��״��)Wͨ��100 mL3 mol/L��Y��ˮ��Һ����Һ�е�������_______________��
����Ȼ���д���X��Z��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100 mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5 mol/L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ g��
�Ȣ�͢���ΪX�����е�����Ԫ���е����ֻ�������ɵĻ������������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬װ�â��в�����ɫ������װ�â��п��ռ���һ����ɫ��ȼ�����塣
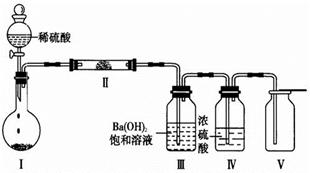
��װ�â������ʵĻ�ѧʽ�� ��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ�â������壬�û�����Ļ�ѧʽ�� ����������װ���� (����ͼѡ���Ҫװ�ã���д���)��

��ش�
��W�Ļ�ѧʽ��____________________��
��X��Y����Һ�з�Ӧ�����ӷ���ʽ��_______________________________��
�Ǣٽ�4.48 L(������Ϊ��״��)Wͨ��100 mL3 mol/L��Y��ˮ��Һ����Һ�е�������_______________��
����Ȼ���д���X��Z��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100 mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5 mol/L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ g��
�Ȣ�͢���ΪX�����е�����Ԫ���е����ֻ�������ɵĻ������������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬װ�â��в�����ɫ������װ�â��п��ռ���һ����ɫ��ȼ�����塣

��װ�â������ʵĻ�ѧʽ�� ��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ�â������壬�û�����Ļ�ѧʽ�� ����������װ���� (����ͼѡ���Ҫװ�ã���д���)��
��CO2��1�֣�
��HCO3-+ OH-= CO32- + H2O��2�֣�
�Ǣ�Na2CO3��NaHCO3 ��2�֣���2.65��2�֣�
�Ȣ� Na2O2��1�֣�
����H2O2 ��1�֣�����������1�֣�
��HCO3-+ OH-= CO32- + H2O��2�֣�
�Ǣ�Na2CO3��NaHCO3 ��2�֣���2.65��2�֣�
�Ȣ� Na2O2��1�֣�
����H2O2 ��1�֣�����������1�֣�
��

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ

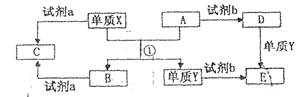

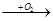 B
B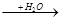 D
D Bת���Ļ�ѧ����ʽ�� ��
Bת���Ļ�ѧ����ʽ�� ��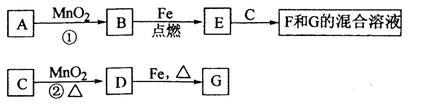
 ��
��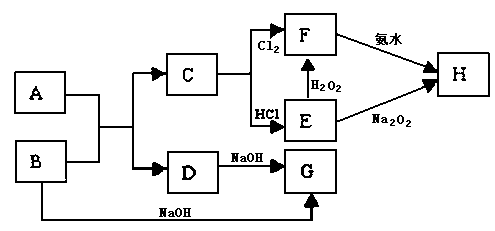
 �����ʹ���������ӷ���ʽ�� ��
�����ʹ���������ӷ���ʽ�� ��