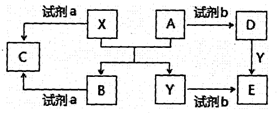
��Ŀ����
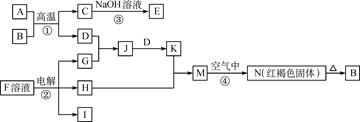
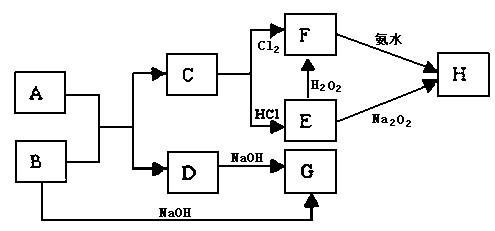
��15�֣���ѧ��ѧ�м��ֳ������ʵ�ת����ϵ��ͼ��ʾ������֪��F�ı�����Һ�����ˮ�У���пɵõ���HΪ��ɢ�ʵĺ��ɫ���塣
��ش��������⣺
��1�����ɫ������H���ӵ�ֱ����С��Χ ��
��2��д��D��һ�ֹ�ҵ��;�� ��
��3����д��H2O2�ĵ���ʽ�� ��
��д��B��G�����ӷ���ʽ ��
��4����A��Bǡ����ȫ��Ӧ��������C���������ᷴӦ�õ�a mol���壬��ȡ������B���������ᷴӦ�õ�b mol���壬a:b=5:7����A�Ļ�ѧʽΪ ��
��5����ʯī���缫�����G���������������Һ������ͼ�������������� �ݡ��������һ��ʱ�䣬��X����������Һ�л��ɹ۲쵽������ ��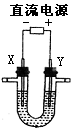 �����ʹ���������ӷ���ʽ�� ��
�����ʹ���������ӷ���ʽ�� ��
�Ͽ���Դ�����������Һ�����ձ��г�ֽ��裬������ ��ԭ���� ��

��ش��������⣺
��1�����ɫ������H���ӵ�ֱ����С��Χ ��
��2��д��D��һ�ֹ�ҵ��;�� ��
��3����д��H2O2�ĵ���ʽ�� ��
��д��B��G�����ӷ���ʽ ��
��4����A��Bǡ����ȫ��Ӧ��������C���������ᷴӦ�õ�a mol���壬��ȡ������B���������ᷴӦ�õ�b mol���壬a:b=5:7����A�Ļ�ѧʽΪ ��
��5����ʯī���缫�����G���������������Һ������ͼ�������������� �ݡ��������һ��ʱ�䣬��X����������Һ�л��ɹ۲쵽������ ��
 �����ʹ���������ӷ���ʽ�� ��
�����ʹ���������ӷ���ʽ�� ���Ͽ���Դ�����������Һ�����ձ��г�ֽ��裬������ ��ԭ���� ��
��15�֣�
��1��1nm ~ 100nm ��1�֣�
��2�����ұ���������²��ϵȣ�����������Ҳ�ɣ���1�֣�
��3�� �� ����2�֣���2Al+2OH-+2H2O=2AlO2-+3H2����2�֣�
����2�֣���2Al+2OH-+2H2O=2AlO2-+3H2����2�֣�
��4��Fe5O7����2�֣�
��5���ȳ��ְ�ɫ���ǣ����ֱ���� ��2�֣� Al3++3OH-=3Al(OH)3����Al(OH)3+ OH-= AlO2-+2H2O����1�֣����ְ�ɫ���� ��1�֣� ��������Al3+�����������ɵ�AlO2-ǡ�÷�Ӧ����Al(OH)3�����ߴ𡰸����ܷ�Ӧ��2AlCl3+ 6H2O 2Al(OH)3+3H2��+3Cl2�������ǡ������Al(OH)3����������÷֣���2�֣�
2Al(OH)3+3H2��+3Cl2�������ǡ������Al(OH)3����������÷֣���2�֣�
��1��1nm ~ 100nm ��1�֣�
��2�����ұ���������²��ϵȣ�����������Ҳ�ɣ���1�֣�
��3�� ��
 ����2�֣���2Al+2OH-+2H2O=2AlO2-+3H2����2�֣�
����2�֣���2Al+2OH-+2H2O=2AlO2-+3H2����2�֣���4��Fe5O7����2�֣�
��5���ȳ��ְ�ɫ���ǣ����ֱ���� ��2�֣� Al3++3OH-=3Al(OH)3����Al(OH)3+ OH-= AlO2-+2H2O����1�֣����ְ�ɫ���� ��1�֣� ��������Al3+�����������ɵ�AlO2-ǡ�÷�Ӧ����Al(OH)3�����ߴ𡰸����ܷ�Ӧ��2AlCl3+ 6H2O
 2Al(OH)3+3H2��+3Cl2�������ǡ������Al(OH)3����������÷֣���2�֣�
2Al(OH)3+3H2��+3Cl2�������ǡ������Al(OH)3����������÷֣���2�֣���

��ϰ��ϵ�д�
�����Ŀ