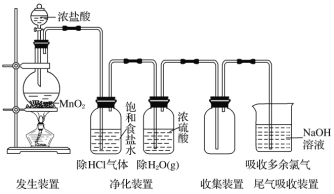
��Ŀ����
����Ŀ��H2O2��Ϊ��ɫ��������Ӧ���ڷ�ˮ��������ֽ�ͻ�ѧ�ϳɵ���ҵ��
��1����֪��H2(g)+![]() O2(g)=H2O(l) ��H1=��285.8kJ��mol-1
O2(g)=H2O(l) ��H1=��285.8kJ��mol-1
H2(g)+O2(g)=H2O2(l) ��H2=��135.8kJ��mol-1
��H2(g)��O2(g)�ķ�Ӧ�У�������ѧ�ϸ������IJ�����__��ԭ����__��
�ڳ����£�H2O2�ֽ���Ȼ�ѧ����ʽΪ__��
��2���ҹ���ѧ��ʹ��Ag9�Ŵ����������о�H2O2�ĺϳɡ�������Ļ�ܺͷ�Ӧ�ȣ������ʾ�����ü����ģ�ⷴӦ������ͼ��ʾ��TS��ʾ����̬����ʾ���������������֣���
Ag9�Ŵ�������H2O2�Ļ��Ea�ͷ�Ӧ��![]()
���� | ����̬ | Ea/kJ |
| |
A | Ag9 | TS1 | 74.1 | +68.7 |
B | H��Ag9 | TS2 | 108.7 | -27.2 |
C | H��Ag9��H+ O2 | TS3 | 78.4 | -75.4 |
D | HOO | TS4 | 124.7 | +31.3 |

��ͨ�����Ͳ���___������ĸ�������ݣ���ܣ������Խϴ������ߺϳɷ�Ӧ�����ʡ�
�ڷ�Ӧ������2��3���ѵĻ�ѧ��Ϊ___������ţ���
A.O2������� B.H2������ C.Ag9OOH������
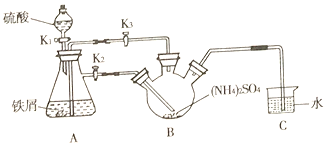
��3������������ͬ���ŵ����H2O2�������[(NH4)2S2O8]��ԭ����ͼ��ʾ�������Ϸŵ��������___�������ĵ缫��ӦʽΪ___��

��4�������£�H2O2�ֽ����ʷ���v=0.0625��c(H2O2)mg��L-1��s-1��c(H2O2)��ʱ��仯���±���
C(H2O2) (mg | 10000.0 | 8000.0 | 4000.0 | 2000.0 | 1000.0 |
�ֽ�ʱ��(s) | 0 | 7 | 23 | 39 | 55 |
�ٵ�c(H2O2)=8000.0mg��L-1ʱ��v=__mg��L-1��s-1��
�ڵ�c(H2O2)��Ϊ5000.0mg��L-1ʱ���ֽ�ʱ��Ϊ___s��
���𰸡�H2O ��S����С��������H1����H2��С������H2O�ķ�Ӧ���Ƹ��� 2H2O2(l)=2H2O(l)+O2(g) ��H=-300.0kJ��mol-1 D AB SO42- O2+2H++2e-=H2O2 500 16
��������
����ͨ��![]() ���жϷ�Ӧ�����ƣ���ѧ��Ӧ�������ɷ�Ӧ���������IJ�������ģ����ݵ���װ�ã����������������ʧ��������S2O82-����Ԫ�ػ��ϼ۽��ͣ������������������ɡ�
���жϷ�Ӧ�����ƣ���ѧ��Ӧ�������ɷ�Ӧ���������IJ�������ģ����ݵ���װ�ã����������������ʧ��������S2O82-����Ԫ�ػ��ϼ۽��ͣ������������������ɡ�
��1���ٴ�����ѧ����������ͬ��Ӧ�ﷴӦʱ������Ӧ����S����С��������H1����H2��С������![]() ������H2O�ķ�Ӧ���Ƹ���
������H2O�ķ�Ӧ���Ƹ���
�ڸ��ݸ�˹���ɣ��ɷ�Ӧ1-��Ӧ2�ɵó�H2O2�ֽ���Ȼ�ѧ����ʽΪ2H2O2(l)=2H2O(l)+O2(g) ��H=-300.0kJ��mol-1��
��2������Ҫ�ϴ������ߺϳɷ�Ӧ�����ʣ�ȡ���ڷ�Ӧ�ľ�����Ӧ���ʵIJ��裬�ǾͿ�ÿ����Ӧ�Ļ�ܵĴ�С���ɱ������ݿ�֪����D�Ļ��������ͨ�����Ͳ���D�����ݣ���ܣ������Խϴ������ߺϳɷ�Ӧ�����ʡ�
�ڷ�Ӧ������2��3��Ӧ�ķ���ʽΪAg9O2+H2![]() H-Ag9OOH����֪O2����������H2������������ѡ�
H-Ag9OOH����֪O2����������H2������������ѡ�
��3�����ݵ���װ�ÿ�֪����Ԫ�ػ��ϼ����ߣ������������������ʧ��������S2O82-��������������ԭ��Ӧ�������õ��������������ɹ������⣬�缫��ӦʽΪO2+2H++2e-=H2O2��
��4���ٵ�c(H2O2)=8000.0mg��L-1ʱ���ֽ�����v=0.0625��c(H2O2)mg��L-1��s-1=��0.0625![]() 8000.0��mg��L-1��s-1=500 mg��L-1��s-1��
8000.0��mg��L-1��s-1=500 mg��L-1��s-1��
���Աȹ�������Ũ�ȼ���һ������ʱ��֮���ϵ�����Է��ֹ�������Ũ�ȼ���һ�����õ�ʱ��Ϊ��ֵ����Ϊ16s���ʵ�c(H2O2)��10000 mg��L-1��Ϊ5000.0mg��L-1ʱ���ֽ�ʱ��Ϊ16s��

����Ŀ������β���������з����ķ�ӦΪ2NO(g)+2CO(g)![]() N2(g)+2 CO2(g)��һ���¶��£� �����������Ϊ1.0L�����ܱ������з���������Ӧ������й�ʵ���������£�
N2(g)+2 CO2(g)��һ���¶��£� �����������Ϊ1.0L�����ܱ������з���������Ӧ������й�ʵ���������£�
���� | �¶�/���棩 | ��ʼ���ʵ�����mol) | ƽ�����ʵ�����mol) | ||||
NO | CO | N2 | CO2 | N2 | CO2 | ||
I | 400 | 0.2 | 0.2 | 0 | 0 | 0.12 | |
II | 400 | 0.4 | 0.4 | 0 | 0 | ||
III | 300 | 0 | 0 | 0.1 | 0.2 | 0.075 | |
����˵����ȷ��
A. �÷�Ӧ�Ħ�S<0����H<0
B. ����I�дﵽƽ������ʱ��2s����v(N2)=0.06 molL-1��S-1
C. �ﵽƽ��ʱ����ϵ��c(CO)��ϵ��c(CO,����II)>2c(CO������I)
D. ����ʼʱ��I�г���NO��CO��N2��CO2��0.1mol����ʼʱV��>V��
����Ŀ��T��ʱ����2L���ܱ������У�����X��Y��Z�����ʵ�����ʱ��ı仯������ͼ��ʾ��
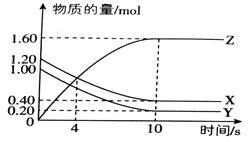
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________.
��2��0~10s�ڣ�X�Ļ�ѧ��Ӧ����Ϊ___________________.
��3���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�������ʾ��
T/�� | 100 | 220 | 830 | 1000 | 1200 |
K | 45.00 | 32.00 | 1.00 | 0.60 | 0.38 |
��÷�Ӧ�Ħ�H__________0 (�>������<����=��)
��4��830��ʱ�����ݻ�Ϊ10L�ĺ����ܱ������г���5molX���塢7.8molY�����7.1mol Z���壬��ʱ��(��)_______��(��) (�>������<����=��)
��5����ͼ��ʾ��Ӧ���¶�Ϊ_________________��