��Ŀ����
����Ŀ��ʵ���ҳ�����������Ϊ36.5%�����ᣨ�ܶ�Ϊ1.20g/�M3������480mL1.0mol/LHCl���ش��������⣺
(1)��Ũ��������ʵ���Ũ����________________
(2)����ʱ����Ҫ�IJ�����������Ͳ���ձ�������������ͷ�ιܡ�____________________���������������õ��������������÷ֱ��� ___________________��________________________��
(3)��ʵ���������������Һ��Ũ��ƫ�ߵ��� __________________________________________
A����ȡŨ����ʱ������Ͳ�̶�
B��ҡ�Ⱥ���Һ����ڿ̶��ߣ��μ�����ˮ���̶�����ҡ��
C������ʱ��������ƿ�Ŀ̶���
D������ƿ�ڱڸ���ˮ���δ���ﴦ��
E��δ��ȴ�����¾Ͷ���
(4)��ȡ50.0mL��������õ���Һ������һ150mL0.2mol/L��HCl��Һ��ϣ����õ�����Һ�����ʵ���Ũ��Ϊ_________________������Һ������仯�ɺ��ԣ���
���𰸡�12mol/L 500ml����ƿ ���� ���� ACE 0.4mol/L
��������
(1)��Ũ��������ʵ���Ũ�ȿ����ù�ʽ![]() ���м��㣻
���м��㣻
(2)����ʱ����Ҫ�IJ�������������ƿ��������������ͨ��Ϊ���衢������
(3)����ʵ��������ʱ���������ڹ�ʽ![]() ��ͨ���Ը�ʵ������ķ�������ȷ����������n��V���ĸ��������仯�����������ı仯���Ӷ�ȷ����������
��ͨ���Ը�ʵ������ķ�������ȷ����������n��V���ĸ��������仯�����������ı仯���Ӷ�ȷ����������
(4)���ù�ʽ![]() ���м��㡣
���м��㡣
(1)��Ũ��������ʵ���Ũ����![]() mol/L��
mol/L��
�𰸣�12mol/L��
(2)����ʱ����Ҫ�IJ�����������Ͳ���ձ�������������ͷ�ιܡ�500ml����ƿ���������������õ�����������һ��ΪŨ�����ˮϡ��ʱ���������������ǽ��裻�ڶ���Ϊ��ϡ�ͺ������ת��������ƿʱ����������������������
�𰸣����裻������
(3) A����ȡŨ����ʱ������Ͳ�̶ȣ���ȡ��Ũ��������ƫ��������Һ��Ũ��ƫ�ߣ�A�������⣻
B��ҡ�Ⱥ���Һ����ڿ̶��ߣ��μ�����ˮ���̶�����ҡ�ȣ���Һ���ƫ��������Һ��Ũ��ƫ�ͣ�B�������⣻
C������ʱ��������ƿ�Ŀ̶��ߣ���Һ�����ƫС�����������Ũ��ƫ�ߣ�C�������⣻
D������ƿ�ڱڸ���ˮ���δ���ﴦ��������Һ��Ũ�Ȳ�����Ӱ�죬D���������⣻
E��δ��ȴ�����¾Ͷ��ݣ���Һ�����ƫС��������Һ�����ƫ�ߣ�E�������⡣
�𰸣�ACE��
(4) ![]() ��
��
�𰸣�0.4ml/L��

����Ŀ��̼�͵��Ļ����������������й㷺���ڡ��ش���������:
��1�����Ȼ�����NCl3����һ�ֻ�ɫ����״�����д̼�����ζ�Ļӷ����ж�Һ�壬��ԭ�Ӿ�����8e���ṹ��д�������ʽ_____________________���ȼҵ����ʱ������ʳ��ˮ��ͨ����������NH4Cl�����������������ɵ�������Ӧ�����������Ȼ������÷�Ӧ�Ļ�ѧ����ʽΪ______________��
��2����֪��2CO2(g) + 3NaOH(aq)=NaHCO3(aq)+Na2CO3(aq)+H2O(l) ��H=��4a kJ/mol
CO2(g) +2NaOH(aq)=Na2CO3(aq)+H2O(l) ��H=��b kJ/mol
��������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ��Ӧ����ʽΪ:____________��
��3������CO���Խ�NOת��Ϊ����N2���䷴ӦΪ��2NO(g)+2CO(g)![]() N2(g)+2CO2(g)�����ݻ���ΪIL�ļס��ҡ����������£���Ӧ�¶ȷֱ�Ϊ300�桢T�桢300�棩�����зֱ������ͬ��NO��CO����ø�������n(CO)�淴Ӧʱ��t�ı仯������±���ʾ:
N2(g)+2CO2(g)�����ݻ���ΪIL�ļס��ҡ����������£���Ӧ�¶ȷֱ�Ϊ300�桢T�桢300�棩�����зֱ������ͬ��NO��CO����ø�������n(CO)�淴Ӧʱ��t�ı仯������±���ʾ:
t/min | 0 | 40 | 80 | 120 | 160 |
n(CO)����������/mol | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
n(CO)����������/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
n(CO)����������/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
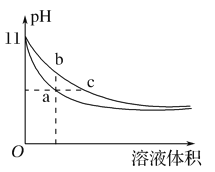
�ټ������У�0��40min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=_______��
�ڸ÷�Ӧ�ġ�H______0���>����<������
�ۼס��������ﵽƽ��ʱ��CO��ת����Ϊ��(��)________��(��)���>��,��< ������������
��4�������£���(NH4)2C2O4��Һ�У���ӦNH4++C2O42��+H2O![]() NH3��H2O+HC2O4����ƽ�ⳣ��K=___������֪�����£�H2C2O4�ĵ���ƽ�ⳣ��Ka1��5��10-2��Ka2��5��10-5 H2O�ĵ���ƽ�ⳣ��Kw=1��10-14 NH3��H2O�ĵ���ƽ�ⳣ��Kb��2��10-5��
NH3��H2O+HC2O4����ƽ�ⳣ��K=___������֪�����£�H2C2O4�ĵ���ƽ�ⳣ��Ka1��5��10-2��Ka2��5��10-5 H2O�ĵ���ƽ�ⳣ��Kw=1��10-14 NH3��H2O�ĵ���ƽ�ⳣ��Kb��2��10-5��
����Ŀ�������ü�������NO2��Ⱦ�����о���CH4+2NO2![]() N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ�����
N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ�����
��� | �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
�� | T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
�� | T2 | n(CH4) | 0.50 | 0.30 | 0.18 | �� | 0.15 |
����˵����ȷ���ǣ� ��
A. �����У�0~20min�ڣ�NO2�Ľ�������Ϊ0.0125mol��L-1��min-1
B. ��ʵ�����ݿ�֪�÷�Ӧ�����˵��¶�ΪT1
C. 40minʱ��������T2Ӧ�������Ϊ0.18
D. 0��10min�ڣ�CH4�Ľ������ʢ�>��