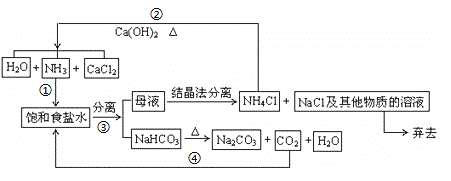
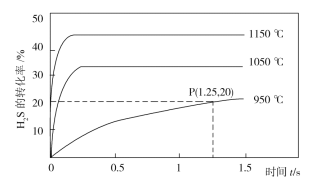
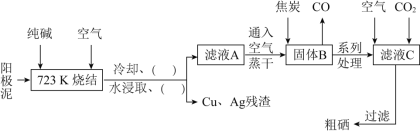��Ŀ����
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��
��![]() ��
��
����AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ��ʾ���ش��������⣺
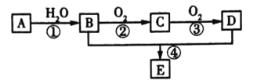
(1)д���������ʵĹ��������ƣ�B_____��C______��
(2)��Ӧ�ܵĻ�ѧ����ʽΪ_____________����Ӧ������__________��
(3)ijѧϰС�����B�Ĵ�������ʵ��װ����ͼ��ʾ���Իش��������⡣
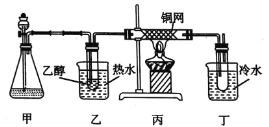
�ټ���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ_______(����ĸ)��
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_____________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������������������ͭ����Һ��Ϻ���ȣ�����Ϊ___________��
���𰸡��ǻ� �Ȼ� ![]() ȡ����Ӧ AD
ȡ����Ӧ AD ![]() ����ש��ɫ����
����ש��ɫ����
��������
A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ����B��BΪCH3CH2OH���Ҵ���������C��CΪCH3CHO��CH3CHO��һ�������ɵ�D��DΪCH3COOH��E��һ����ɫ��������ζ����״Һ�壬CH3COOH��CH3CH2OH����������Ӧ����E��EΪCH3COOCH2CH3������л���Ľṹ�����ʷ������
(1)BΪCH3CH2OH��DΪCH3COOH��B��D�����еĹ����ŷֱ��ǣ��ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
(2)��Ӧ���������������Ʊ�����Ӧ�Ļ�ѧ����ʽ�ǣ�CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O������ȡ����Ӧ(��������Ӧ)���ʴ�Ϊ��CH3COOH+HOCH2CH3
CH3COOCH2CH3+H2O������ȡ����Ӧ(��������Ӧ)���ʴ�Ϊ��CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O��ȡ����Ӧ(��������Ӧ)��
CH3COOCH2CH3+H2O��ȡ����Ӧ(��������Ӧ)��
(3)���Ҵ��Ĵ�������ʵ���У�����װ��ͼ��֪��װ�ü����ڲ���������������˫��ˮ�Ͷ������̣�Ҳ�����ù������ƺ�ˮ��Ӧ��װ������ˮԡ���Ȳ����Ҵ���������������Ϻ�ͨ����װ���е�ͭ������������װ������ˮ��ȴ�õ����
�ٸ�������ķ�����֪��װ�ü����ڲ���������������˫��ˮ�Ͷ������̣�Ҳ�����ù������ƺ�ˮ��Ӧ����ѡAD��
��ʵ������У���װ��Ӳ�ʲ�������ͭ�����������Ҵ�����������ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
���Ҵ�����������ȩ����ȩ������������ͭ����Һ�м��Ȼ����ש��ɫ�������ʴ�Ϊ������ש��ɫ������