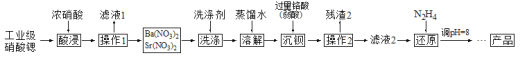
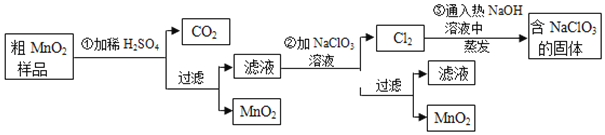
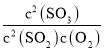��Ŀ����
����Ŀ��ij�����������Ļ�ѧʽ�ɱ�ʾΪKa[Feb(C2O4)c]��xH2O��Ϊ�ⶨ����ɣ���������ʵ�飺
����1����ȡ1.9640g������ᄃ�壬���Ƴ�250.00mL��Һ��
����2��ȡ������Һ25.00mL����ƿ�У�����1mol��L-1����5.0mL�����ȵ�70~85������0.0100mol��L-1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ48.00mL��
����3����Ӧ�����Һ�м���һ����п�ۡ���������ɫǡ����ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����4��������0.0100mol��L-1KMnO4��Һ�ζ�����3������Һ���յ㣬����KMnO4��Һ8.00mL��
(1)����2�У�KMnO4��C2O![]() ����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
(2)����3�л�ɫ��ʧ��ԭ����___(�����ӷ���ʽ��ʾ)��
(3)�����������Һ�Ĺ����У�������ʱ��������ƿ�Ŀ̶��ߣ����������þ��������ˮ�ĺ���___(����ƫ������ƫС��������Ӱ����)��
(4)ͨ������ȷ���������������Ļ�ѧʽ___(д���������)��
���𰸡�5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O 2Fe3++Zn=2Fe2++Zn2+ ƫС ���ݵζ���Ӧ5Fe2++MnO
+16H+=10CO2��+2Mn2++8H2O 2Fe3++Zn=2Fe2++Zn2+ ƫС ���ݵζ���Ӧ5Fe2++MnO![]() +8H+=5Fe3++Mn2++4H2O��250.00mL��Һ�и��������ʵ����ֱ�Ϊ��
+8H+=5Fe3++Mn2++4H2O��250.00mL��Һ�и��������ʵ����ֱ�Ϊ��
n(C2O![]() )=
)=![]() n(MnO
n(MnO![]() )��10=
)��10=![]() =0.012mol��
=0.012mol��
n(Fe3+)=n(Fe2+)=5n(MnO![]() )��10=5��0.0100mol/L��0.008L��10=0.004mol��
)��10=5��0.0100mol/L��0.008L��10=0.004mol��
���ݵ���غ��֪n(K+)=2��0.012mol-3��0.004mol=0.012mol��
���������غ�ɵ�n(H2O)=![]() =0.012mol��
=0.012mol��
n(C2O![]() )��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
)��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
��ѧʽΪK3[Fe(C2O4)3]��3H2O��
��������
ȡ��Ʒ���Ƴ�250.00mL��Һ��ȡ25.00mL���еζ�������Һ�ữ�����ø�����ر�Һ��C2O![]() �������������ĵĸ�����ص���ȷ����Ʒ�в����������֮�����п�۽���������ԭ���������ӣ������ø�����ر�Һ��Fe2+�������Ӷ�ȷ����Ʒ��FeԪ�ص������ٸ��ݵ���غ�ȷ�������ӵ��������������غ�ȷ��ˮ������
�������������ĵĸ�����ص���ȷ����Ʒ�в����������֮�����п�۽���������ԭ���������ӣ������ø�����ر�Һ��Fe2+�������Ӷ�ȷ����Ʒ��FeԪ�ص������ٸ��ݵ���غ�ȷ�������ӵ��������������غ�ȷ��ˮ������
(1)KMnO4��C2O![]() ����ΪCO2����������ԭ��Mn2+�����ݵ����غ�͵���غ�ɵ����ӷ���ʽΪ5C2O
����ΪCO2����������ԭ��Mn2+�����ݵ����غ�͵���غ�ɵ����ӷ���ʽΪ5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O��
+16H+=10CO2��+2Mn2++8H2O��
(2)��Һ�������Fe3+���ʻ�ɫ������п�ۺ������ӷ�Ӧʹ��ɫ��ʧ�����ӷ���ʽΪ2Fe3++Zn=2Fe2++Zn2+��
(3)����ʱ��������ƿ�Ŀ̶��ᵼ�����Ƶ���ƷŨ��ƫ��ͨ���ζ���ȷ����C2O![]() ��Fe3+��K+����ƫ��ȷ��ˮ����ʱ��Ҫ����������ȥ�����������ӵ��������Իᵼ����Ʒ��ˮ�ĺ���ƫС��
��Fe3+��K+����ƫ��ȷ��ˮ����ʱ��Ҫ����������ȥ�����������ӵ��������Իᵼ����Ʒ��ˮ�ĺ���ƫС��
(4)���ݷ�Ӧ5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O��֪��250.00mL��Һ��n(C2O
+16H+=10CO2��+2Mn2++8H2O��֪��250.00mL��Һ��n(C2O![]() )=
)=![]() n(MnO
n(MnO![]() )��10=
)��10=![]() =0.012mol��
=0.012mol��
MnO![]() ����Fe2+ʱ��MnO
����Fe2+ʱ��MnO![]() ת��ΪMn2+�����ϼ۽���5�ۣ�Fe2+ת��ΪFe3+�����ϼ�����1�ۣ����Զ��ߵ�ϵ����Ϊ5:1������n(Fe3+)=n(Fe2+)=5n(MnO
ת��ΪMn2+�����ϼ۽���5�ۣ�Fe2+ת��ΪFe3+�����ϼ�����1�ۣ����Զ��ߵ�ϵ����Ϊ5:1������n(Fe3+)=n(Fe2+)=5n(MnO![]() )��10=5��0.0100mol/L��0.008L��10=0.004mol��
)��10=5��0.0100mol/L��0.008L��10=0.004mol��
���ݵ���غ��֪n(K+)=2��0.012mol-3��0.004mol=0.012mol��
���������غ�ɵ�n(H2O)=![]() =0.012mol��
=0.012mol��
n(C2O![]() )��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
)��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
��ѧʽΪK3[Fe(C2O4)3]��3H2O��

 ��У����ϵ�д�
��У����ϵ�д�