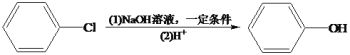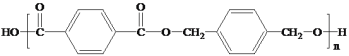��Ŀ����
����Ŀ������������������ͿƼ�������ء��ش��������⣺
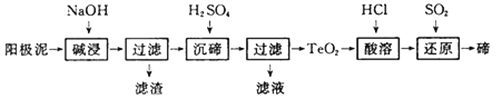
(1)���ǽ������������ǵ�������ϵ���С��ҹ����ж���֪ʶ��Ȩ�ĵ���оƬ����оһ�š�����ҹ������ʷ�Ͽհף�����оһ�š����ϵĻ�ѧʽΪ______����ͳ�����ǽ����������ճ����������Ź㷺�����á�������ˮ��������о��õ���ԭ�ϵ�������____������̼�����ڲ�����¯�з�Ӧ�Ļ�ѧ����ʽΪ____________��
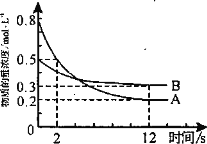
(2)�������ϵ�Ӧ�ø��ǹ㷺����ҵ����30��FeCl3��Һ��ʴ��Ե����ͭ��������ӡˢ��·�塣��ʴ��Һ�к���Fe3����Fe2����Cu2�����ӡ�����ʴҺ�м���������Fe�ۣ�һ�������ڵ�������________����ʵ���ж���ʴҺ�к���Fe2������______________��
���𰸡�Si ʯ��ʯ Na2CO3 �� SiO2![]() Na2SiO3 Fe3����Cu2�� ���Թ�ȡ������ʴҺ�μ���������KMnO4��Һ���Ϻ�ɫ��ɫ��˵������Fe2�������Թ�ȡ������ʴҺ�μӼ���KSCN��Һ������ɫ���ٵμӼ�����ˮ����Һ��ΪѪ��ɫ
Na2SiO3 Fe3����Cu2�� ���Թ�ȡ������ʴҺ�μ���������KMnO4��Һ���Ϻ�ɫ��ɫ��˵������Fe2�������Թ�ȡ������ʴҺ�μӼ���KSCN��Һ������ɫ���ٵμӼ�����ˮ����Һ��ΪѪ��ɫ
��������
(1 )���dz��õİ뵼�壬����оһ��"���ϵĻ�ѧʽΪSi������������������ʯ��ʯ�������ʯӢ������ˮ���ԭ����ʯ��ʯ���������ԭ�ϣ���������о��õ���ԭ�ϵ�������ʯ��ʯ�� ����̼�����ڲ�����¯�з�Ӧ�Ļ�ѧ����ʽΪNa2CO3+SiO2![]() Na2SiO3+CO2����
Na2SiO3+CO2����
(2 )��ʴ��Һ�к���Fe3+��Fe2+��Cu2+ ���ӣ�����ʴҺ�м���������Fe�ۣ������ӡ�ͭ���Ӿ�����ԭ�����һ�������ڵ�������Fe3 +�� Cu2+ ���������Ӿ��л�ԭ�ԣ�������ʵ���ж���ʴҺ�к���Fe2+���ӵ�ʵ�鷽�������Թ�ȡ������ʴҺ�μ���������KMnO4��Һ���Ϻ�ɫ��ɫ��˵������Fe2���������Թ�ȡ������ʴҺ�μӼ���KSCN��Һ������ɫ���ٵμӼ�����ˮ����Һ��ΪѪ��ɫ��˵������Fe2������