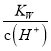МвДҝДЪИЭ
ЎҫМвДҝЎҝФЪt ЎжКұЈ¬AgBrФЪЛ®ЦРөДіБөнИЬҪвЖҪәвЗъПЯИзНјЛщКҫЎЈТСЦӘt ЎжКұAgClөДKspЈҪ4ЎБ10Јӯ10Ј¬ПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)

A. јУИлNaBr№ММеЈ¬AgBrөДИЬҪв¶ИјхРЎЈ¬KspТІјхРЎ
B. ФЪAgBrұҘәНИЬТәЦРјУИл№ММеNaBrЈ¬ҝЙК№ИЬТәЦРcөгұдөҪbөг
C. НјЦРaөг¶ФУҰөДКЗУРAgBrіБөнЙъіЙ
D. ФЪt ЎжКұЈ¬AgCl(s)Ј«BrЈӯ(aq) AgBr(s)Ј«ClЈӯ(aq)өДЖҪәвіЈКэKЎЦ816
Ўҫҙр°ёЎҝD
ЎҫҪвОцЎҝAЈ®KspЦ»УлОВ¶ИУР№ШЈ»BЈ®ФЪAgBrұҘәНИЬТәЦРјУИлNaBr№ММеәуЈ¬c(Br-)ФцҙуЈ¬ИЬҪвЖҪәвДжПтТЖ¶ҜЈ¬c(Ag+)јхРЎЈ»CЈ®НјЦРaөгұнКҫc(Br-)c(Ag+)ЈјKsp(AgBr)Ј»DЈ®ёщҫЭKЁT![]() =
=![]() јЖЛгЎЈ
јЖЛгЎЈ
AЈ®јУИлNaBr№ММеЈ¬AgBrөДИЬҪв¶ИјхРЎЈ¬ө«KspІ»ұдЈ¬№КAҙнОуЈ»BЈ®ФЪAgBrұҘәНИЬТәЦРјУИлNaBr№ММеәуЈ¬c(Br-)ФцҙуЈ¬ИЬҪвЖҪәвДжПтТЖ¶ҜЈ¬c(Ag+)јхРЎЈ¬І»ДЬК№ИЬТәЦРcөгұдөҪbөгЈ¬№КBҙнОуЎЈCЈ®НјЦРaөгұнКҫc(Br-)c(Ag+)ЈјKsp(AgBr)Ј¬Г»УРAgBrіБөнЙъіЙЈ¬№КCҙнОуЈ»DЈ®·ҙУҰAgCl(s)+Br-(aq)AgBr(s)+Cl-(aq)өДЖҪәвіЈКэОӘЈәKЁT![]() =
=![]() =
=![]() ЎЦ816Ј¬№КDХэИ·Ј»№КСЎDЎЈ
ЎЦ816Ј¬№КDХэИ·Ј»№КСЎDЎЈ

ЎҫМвДҝЎҝK2Cr2O7КЗТ»ЦЦЦШТӘөД»Ҝ№ӨФӯБПЎЈТФёхМъҝу(ЦчТӘіЙ·ЦОӘFeOЎӨCr2O3,»№ә¬УРAl2O3ЎўFe2OөИФУЦК)ОӘФӯБПЦЖұёK2Cr2O7өДТ»ЦЦ№ӨТХБчіМИзПВЈә

ТСЦӘЈә(a) Cr2O72-+H2O![]() 2CrO42-+2H+
2CrO42-+2H+
(b)І»Н¬ОВ¶ИПВёчОпЦКөДИЬҪв¶И
Оп ЦК | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
ИЬҪв¶ИЈЁg/100g H2OЈ© | 0Ўж | 28 | 35.7 | 4.7 | 163 |
40Ўж | 40.1 | 36.4 | 26.3 | 215 | |
80Ўж | 51.3 | 38 | 70 | 376 | |
(c)ІҪЦиўЩөДЦчТӘ·ҙУҰОӘЈәFeOCr2O3+Na2CO3+NaNO3![]() Na2CrO4+Fe2O3+CO2+NaNO2ЈЁОҙЕдЖҪЈ©ЎЈ
Na2CrO4+Fe2O3+CO2+NaNO2ЈЁОҙЕдЖҪЈ©ЎЈ
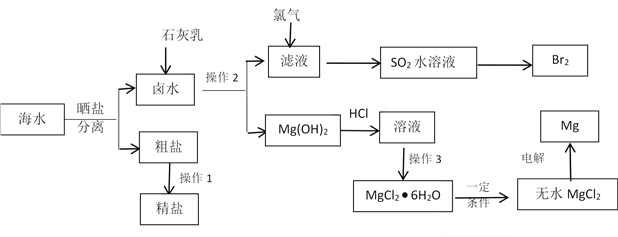
ЈЁ1Ј© Ў°ВЛФь1ЎұәН Ў°ВЛФь2ЎұөДЦчТӘіЙ·Ц·ЦұрКЗ_________Ј¬_________(Мо»ҜС§КҪ).
РҙіцўЫөДАлЧУ·ҙУҰ·ҪіМКҪ________________________________________;
ІҪЦиўЬЦРөчPHҝЙСЎУГТФПВКФјБ__________ЎЈ
AЎўNH3 BЎў KOH CЎўCH3COOH DЎў HCl
ЈЁ2Ј©ФЪІҪЦиўЭЦРјУИлККБҝKClЈ¬____________________Ј¬№эВЛөГөҪK2Cr2O7№ММеЎЈ
ЈЁ3Ј©Ді№Өі§УГakg ёхМъҝу·ЫЈЁә¬Cr2O3 40%Ј©ЦЖK2Cr2O7Ј¬ЧоЦХөГөҪІъЖ· b kgЈ¬ІъВКОӘ_____________________________ЎБ100%ЎЈЈЁБРјЖЛгКҪЈ©ЎЈ
ЈЁ4Ј©»Ҝ»№Фӯ·ЁҝЙіэИҘ·ПЛ®ЦРөДCr2O72-Ј¬ИЎә¬Cr2O72-өДДЈДвЛ®Сщ·ЦұрФЪІ»Н¬PHМхјюПВ,ПтГҝёцЛ®СщЦР·Ц ұрјУТ»¶ЁБҝөДFeSO4ЎўNaHSO3Ј¬ҪБ°иЈ¬ід·Ц·ҙУҰ,И»әуөОјУCa(OH)2РьЧЗТә,ҫІЦГіБөнЈ¬Ів¶ЁёхИҘіэВК,КөСйҪб№ыПВНјЛщКҫЎЈ

ўЩФЪЛбРФМхјюПВЈ¬NaHSO3К№Cr2O72-»№ФӯіЙОӘCr3+,ЗлРҙіцNaHSO3УлCr2O72-·ҙУҰөДАлЧУ·ҪіМКҪ:____________________________________ЎЈ
ўЪ pH>8КұЈ¬СЗМъСО¶Ф+6јЫCrөДИҘіэР§№ы·ҙ¶шПВҪө,ҝЙДЬөДФӯТтКЗ_________________ЎЈ