��Ŀ����
����Ŀ��ijʵ��С�����ʵ�飬��������KMnO4��Һ��H2C2O4��Һ��Ӧ���ⶨ��Һ��ɫ��ʧ����ʱ��ķ������о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졣��ѡ���ʵ��ҩƷ�У�0.01mol��L��1����KMnO4��Һ��0.1mol��L��1����KMnO4��Һ��0.1mol��L��1H2C2O4��Һ��0.2mol��L��1H2C2O4��Һ��
��1��H2C2O4��Һ������KMnO4��Һ��Ӧ�����ӷ���ʽΪ_______________��
��2�����������ʵ����Ʊ���
ʵ�� | ����KMnO4��Һ | H2C2O4��Һ | ��ɫʱ��/s | ||
c/(mol��L��1) | V/mL | c/(mol��L��1) | V/mL | ||
�� | 0.01 | 4 | 0.1 | 2 | t1 |
�� | a | 4 | b | 2 | t2 |
�ٱ���a=________��b=________��
�ڼ���ʵ��KMnO4��ƽ����Ӧ�����ǣ�____________���ú�t1��ʽ�ӱ�ʾ����
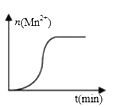
��3�����ij��ʵ�飨���£�ʱ����Һ��Mn2+���ʵ�����ʱ���ϵ��ͼ�������n(Mn2+)�ڷ�Ӧ��ʼʱ�仯����һ��ʱ�����������ԭ��___________________��
���𰸡�5H2C2O4+2MnO4��+6H+��2Mn2++10CO2��+8H2O 0.01 0.2 ![]() Mn2+�Ը÷�Ӧ�д�����
Mn2+�Ը÷�Ӧ�д�����
��������
(1)������ؾ���ǿ�����ԣ��ܰѲ��������ɶ�����̼����������ԭ�ɶ����̣�ͬʱ����ˮ�����ݵ�ʧ�����غ��ԭ���غ���
(2)�ٱȽ���Һ����ɫʱ�䣬�������������Һ��Ũ��Ӧһ�£�H2C2O4��Һ��Ũ��Ӧ��ͬ��
�ڸ���v=![]() ���㣻
���㣻
(3)Ũ�ȡ��¶ȡ�����Ӱ�컯ѧ��Ӧ���ʣ����ݷ�Ӧ5H2C2O4+2KMnO4+3H2SO4��2SO4+2MnSO4+10CO2��+8H2O��֪���÷�Ӧ�в����������ӣ������ӶԸ÷�Ӧ���˴����ã����ӿ��˷�Ӧ���ʡ�
(1)������ؾ���ǿ�����ԣ��Ѳ����е�C��+3��������+4�۵Ķ�����̼��MnԪ�ش�+7�۱仯��+2�۵��̣����ڲ����������2��Cԭ�ӣ����Ը�����������ķ�Ӧ����Ϊ5��2���ʷ�Ӧ�ķ���ʽΪ��5H2C2O4+2MnO4��+6H+��2Mn2++10CO2��+8H2O��
(2)�ٱȽ���Һ����ɫʱ�䣬�������������Һ��Ũ��Ӧһ�£����a=0.01��H2C2O4��Һ��Ũ��Ӧ��ͬ������H2C2O4��Һ��Ũ�ȿ�ѡ��0.2molL-1�����b=0.2��
�ڷ�Ӧ��ʼʱ��c(KMnO4)=![]() =
=![]() molL-1����Ӧʱ�䣺��t=t1s��KMnO4��ƽ����Ӧ���ʣ���(KMnO4)=
molL-1����Ӧʱ�䣺��t=t1s��KMnO4��ƽ����Ӧ���ʣ���(KMnO4)=![]() =
=![]() molL-1s-1��
molL-1s-1��
(3)���ij��ʵ��(����)ʱ��Һ��Mn2+���ʵ�����ʱ���ϵ��ͼ���������ӷ�Ӧ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��֪���÷�Ӧ�в����������ӣ������ӶԸ÷�Ӧ���˴����ã����ӿ��˷�Ӧ���ʣ���n(Mn2+)�ڷ�Ӧ��ʼʱ�仯����һ��ʱ����������

����Ŀ�������½�������ʵ�飬����ʵ��������������õ��Ľ�����ȷ����
ѡ�� | ʵ����������� | ���� |
A | �����£�������Һ��ͨ����CO2����Һ����� | ̼������Աȱ��ӵ�ǿ |
B | �����Ҵ���Ũ����Ļ����Һ��������������ͨ����������KMnO4��Һ����Һ�Ϻ�ɫ��ȥ | ����ϩ���� |
C | ��5 mL 0.1 mol��L1 KI��Һ�м���1 mL 0.1 mol��L1 FeCl3��Һ����ַ�Ӧ����ȡ��Һ����ˮ���еμ�KSCN��Һ����Һ��Ѫ��ɫ | I����Fe3+�ķ�Ӧ��һ���� |
D | ��NaHCO3��Һ�еμ���ɫʯ����Һ����Һ���� | Kw��Ka1(H2CO3)��Ka2(H2CO3) |
A.AB.BC.CD.D