��Ŀ����
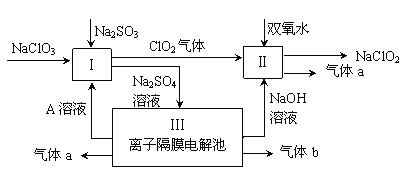
����Ŀ����ij�ϴ���(��ZnO��CuO��Fe2O3��ʯī��MnO2��)�л��ս�������ȡ��������п�Ĺ�����������(��֪��Zn������������������������Al������Ӧ��������������)��
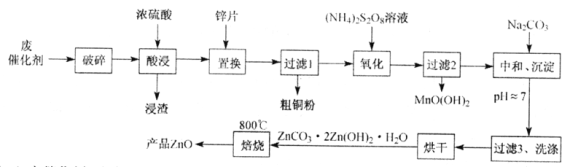
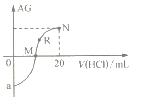
��1���ϴ�������������Ŀ����____________________________________________��
��2����������ʱMn2+�����������ӷ���ʽΪ_________________________________��
��3�����к͡�������ʱ����pH���ߣ����ʽ̼��п�IJ���ƫС��д���䷴Ӧ�����ӷ���ʽ(��дһ������)��____________________________________________��
��4���������ͭ������ͭ������������ʵ�鲽�����£�
I.ȷ��ȡ��ͭ��mg���������������H2O2��Һʹ����ȫ�ܽ⡣
��.����Һ���1~2min����ȥ������H2O2��
��.�������ڱμ��ų�Fe3+�ĸ��š�Ȼ������Թ�����KI��Һ(��Ӧ��2Cu2++4I��=2CuI��+I2)���ټ��뼸�ε�����Һ��ָʾ������ c mol ��L��1Na2S2O3����Һ�ζ�����ɫ��ʧ(I2+2S2O32��=2I��+S4O62��)�Ұ�����ڲ���ɫ��������Na2S2O3����ҺVmL��
��ͭ����������Ϊ______________________��
��ȱ�ٲ��������ʹ��õ�ͭ����������___________(����ƫ��������ƫС��������������)
���𰸡���߽�ȡ�ʺͽ�ȡ���� Mn2����S2O82����3H2O��MnO(OH)2����2SO42����4H�� Zn(OH)2��2OH����ZnO22����2H2O��Zn2����4OH����ZnO22����2H2O��ZnCO3��2Zn(OH)2 �� H2O��4OH����ZnCO3��ZnO22����5H2O 6.4Vc/m% ƫ��
��������
��1���ϴ�������������Ŀ������߽�ȡ�ʺͽ�ȡ���ʣ�
��2����������ʱS2O82���ڼ��������¿��Խ�Mn2+������MnO(OH)2����Ӧ�����ӷ���ʽΪMn2����S2O82����3H2O��MnO(OH)2����2SO42����4H����
��3�����к͡�������ʱ����pH���ߣ����ʽ̼��п�IJ���ƫС��������Ӧ�����ӷ���ʽ��Zn(OH)2��2OH����ZnO22����2H2O��Zn2����4OH����ZnO22����2H2O��ZnCO3��2Zn(OH)2 �� H2O��4OH����ZnCO3��ZnO22����5H2O��
��4���������ͭ������ͭ������������ʵ�鲽�����£�
I.ȷ��ȡ��ͭ��mg���������������H2O2��Һʹ����ȫ�ܽ⡣
��.����Һ���1~2min����ȥ������H2O2��
��.�������ڱμ��ų�Fe3+�ĸ��š�Ȼ������Թ�����KI��Һ(�ټ��뼸�ε�����Һ��ָʾ������ c mol ��L��1Na2S2O3����Һ�ζ�����ɫ��ʧ�Ұ�����ڲ���ɫ��������Na2S2O3����ҺVmL��
�ٸ��ݷ�Ӧ��2Cu2++4I��=2CuI��+I2��I2+2S2O32��=2I��+S4O62����֪��ϵ2Cu2+~~I2~~~2S2O32����ͭ����������Ϊ![]() ��
��
��ȱ�ٲ�����,�������ĵ����ӵ���ƫ�࣬�ζ�ʱ���ĵ�Na2S2O3��ƫ�࣬��ʹ��õ�ͭ����������ƫ��

����Ŀ����֪X��Y��Z��R��QΪ���ڱ���ԭ���������������ǰ36��Ԫ�ء������Ϣ���£�
XԪ������������ḻ��Ԫ�� |
YԪ�ػ�̬ԭ�ӵĺ���p��������s��������1 |
ZԪ�ر���Ϊ��̫�ս�������Ҳ�С����������֮�ƣ����̬ԭ�Ӵ������2��δ�ɶԵ��� |
RԪ����Ԫ�����ڱ��ĵ�ʮһ�� |
QԪ�������ڱ�����RԪ����ͬһ������ |
��ش��������⣺
��1��YX3����ԭ�ӵ��ӻ����������________________�����ӻ�����YX5�ĵ���ʽ��_________��
��2��CO��Y2���ڵȵ����壬1 ��CO�����к��е�������Ŀ��________����
��3����ҵ������ZO2��̼�ᱵ������״̬����ȡ������A(A�ɿ���һ�ֺ�������)��A����ľ���Ϊ������(��ͼ)��
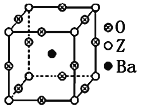
���Ʊ�A�Ļ�ѧ����ʽ��_________________________________________��
����A�����У�Z����λ��Ϊ_______________��
����A�����У�����ZԪ����������������ģ�BaԪ������������Ķ��㣬��OԪ�ش����������______________��
��4��R2+���ӵ���Χ���Ӳ�����Ų�ʽΪ______________��R2O���۵��R2S��________(��ߡ��͡�)��
��5��QԪ�غ���(S)Ԫ���ܹ��γɻ�����B��B����ľ���Ϊ������(��ͼ)���������ⳤΪ540.0 pm�����ܶ�Ϊ___________________________g��cm3(��ʽ������)��
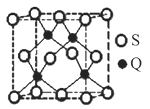 ��
��
����Ŀ������ƽ�ⳣ���Ǻ���������ʵ���̶ȵ�����������֪��
��ѧʽ | ���볣��(25 ��) |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
��1��25 ��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ������Һ��pH�ɴ�С��˳��Ϊ________(�û�ѧʽ��ʾ)��
��2����NaCN��Һ��ͨ��������CO2��������Ӧ�Ļ�ѧ����ʽΪ_____________��
��3��25 ��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH��6������Һ��c(CH3COO��)��c(Na��)��________ mol��L��1(�ȷֵ)��c(CH3COO��)/c(CH3COOH)��________��