��Ŀ����
����Ŀ����1��ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӡ�
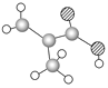
�����ʵĽṹ��ʽΪ__�������������������ŵ�����Ϊ__��
��2�����и������ʣ���O2��O3��H2��D2��T2��12C��14C��CH3CH2CH2CH3��(CH3)2CH2CH3������Ͷ����CH3CH2CH2CH��C2H5��CH3��CH3CH2CH2CH��CH3��C2H5��Ϊͬϵ�����__����Ϊͬ���칹�����__����Ϊͬλ�ص���__����Ϊͬ�����������__����ͬһ���ʵ���__��
��3����̪�dz��õ����ָʾ������ṹ��ʽ��ͼ��ʾ��

�ٷ�̪�ķ���ʽΪ__��
�ڴӽṹ�Ϸ�����̪�ɿ���__��
A.ϩ�� B.���㻯���� C.�������� D.�������� E.�������� F.��������
���𰸡�![]() ̼̼˫�����Ȼ� �� �� �� �� �� C20H14O4 BDF
̼̼˫�����Ȼ� �� �� �� �� �� C20H14O4 BDF
��������
��1���ɷ���ģ�Ϳ�֪���л���Ľṹ��ʽΪ��![]() ���л��ﺬ��̼̼˫�����Ȼ���
���л��ﺬ��̼̼˫�����Ȼ���
��2����O2��O3��Ϊͬ�������壬
��H2��D2��T2��ͬ��Ԫ���γɵĵ��ʣ�
��12C��14C��Ϊͬλ�أ�
��CH3CH2CH2CH3��(CH3)2CH2CH3��Ϊͬ���칹�壬
������Ͷ��黥Ϊͬϵ�
��CH3CH2CH2CH(C2H5)CH3��CH3CH2CH2CH(CH3)C2H5Ϊͬһ�����ʣ�
�ʴ�Ϊ���ݣ��ܣ��ۣ��٣��ޣ�
��3���ٸ��ݷ�̪�Ľṹ��ʽ��֪����ʽΪC20H14O4��
�ڷ�̪�к��з��ǻ������������������зӡ������������ʣ����Կ��Կ��������廯������ࡢ���࣬�ʴ�Ϊ��BDF��