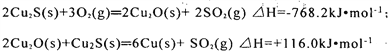��Ŀ����
����Ŀ���±��е��������ƻ�1 mol�����еĻ�ѧ�������ĵ�����(kJ)��

��1���������ʱ������е�������͵�����____��
A��H2 B��Cl2 C��Br2 D��I2
��2�������⻯���У����ȶ�������____��
A��HCl B��HBr C��HI
��3��X2��H2===2HX(X����Cl��Br��I)�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ�� _____________��
��4����ͬ�����£�X2(X����Cl��Br��I)�ֱ���������Ӧ�������ĵ����ʵ���������ʱ���ų������յ�����������________��
���𰸡� A A ���ȷ�Ӧ Cl2
��������(1)����ͼ�����ݿ�֪���ƻ�1mol�����е�����������ĵ�������ߣ���˵���������ȶ������е�������ͣ�A��ȷ����ȷ�𰸣�A��
��2�����ݱ������ݿ�֪���ƻ�1mol�Ȼ����е����ȼ������ĵ�������ߣ���˵���Ȼ������ȶ���A��ȷ����ȷ�𰸣�A��
��3�����ݷ�Ӧ��X2+H2=2HX��H=��Ӧ��ϼ����յ�������-������ɼ��ų�����������������ֵ���м��㣬�������H<0������±�ص����������Ļ��Ϸ�Ӧ��Ϊ���ȷ�Ӧ����ȷ�������ȷ�Ӧ��
��4��������Խ�ȶ����ų�������Խ�������Ȼ������廯�����⻯�������Ȼ������ȶ�����Ϊͬ����Ԫ�ش��ϵ���Ԫ�ص��⻯���ȶ���������X2(X����Cl��Br��I)�ֱ���������Ӧ�������ĵ����ʵ���������ʱ���ų�����������������ȷ�𰸣�Cl2��

 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�����Ŀ������ѡ���У��������ʵ�����ģ������������ޣ��ܹ������Ӧʵ�����
ѡ�� | ʵ������ | ��Ӧʵ�� |
A | ̽����ѧ��Ӧ���� | ȡ5mL0��1mol/LKI��Һ���μ�0��1mol/L FeCl3��Һ5~6�Σ��ɸ�����Һ�мȺ�I2�ֺ���I-��ʵ����ʵ�жϸ÷�Ӧ�ǿ��淴Ӧ |
B | ̽�����ԣ�HCO 3 -��Al(OH) 3 | ��NaHCO3��Һ�еμ�NaAlO2��Һ���۲��Ƿ��а�ɫ�������� |
C | ̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� | ����ֻ�Թܸ�ȡ5mL0��1mol/LKMnO4��Һ���ֱ����2mL0��1mol/L��1mL0��2mol/LH 2C 2O 4��Һ����¼��Һ��ɫ����Ҫ��ʱ�� |
D | ̽�������ԣ�Cl 2��Br 2��I2 | ��NaBr��Һ�еμ���ˮ���ټ������KI��Һ����Һ���� |
A. A B. B C. C D. D
����Ŀ���п�Ժһ�����³ɹ�ʵ���˼����Ч������ϩ�������ڴ����������⣬�������о����ɻ�ż����Ӧ������ϩ����ͼ��ʾ��
���� | ȼ������kJ/mol�� |
���� | 285��8 |
���� | 890��3 |
��ϩ | 1411��5 |
��1����֪������ʵ�ȼ�������ϱ���д�������Ʊ���ϩ���Ȼ�ѧ����ʽ_________��
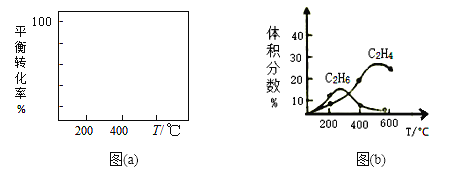
��2����400��ʱ����1L�ĺ��ݷ�Ӧ���г���1molCH4������������Ӧ�����ƽ����������C2H4���������Ϊ20.0 %�����ڸ��¶��£���ƽ�ⳣ��K=_______������ѧƽ���ƶ�ԭ������ͼ(a)�л����÷�Ӧ��ƽ��ת�������¶ȼ�ѹǿ(p1>p2)�Ĺ�ϵ���ߡ�_______________________
��3�����Ʊ�C2H4ʱ��ͨ�����ڸ���Ӧ��2CH4(g)![]() C2H6(g)+H2(g)���ڳ����£������Ϊ1L�ĺ��ݷ�Ӧ���г���1molCH4��Ȼ�������¶ȣ��õ�ͼ(b)��
C2H6(g)+H2(g)���ڳ����£������Ϊ1L�ĺ��ݷ�Ӧ���г���1molCH4��Ȼ�������¶ȣ��õ�ͼ(b)��
����200��ʱ����������������ϩ�����Ҫԭ����_________________________��
����600������ϩ������������ٵ���Ҫԭ����__________________________��
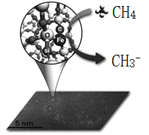
��4����ҵ�ϳ����ó���Ч�ʸߵ�����-������Ϸ�����ȥ��Ȼ���е���������H2S����ת��Ϊ�ɻ������õĵ�������װ������ͼ��ʾ��
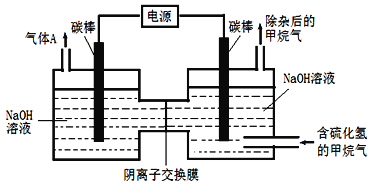
ͨ��ǰ����ͨ��һ��ʱ�京H2S�ļ�������ʹ����NaOH����H2Sת��ΪNa2S���ٽ�ͨ��Դ������ͨ�뺬���ʵļ������������ƺ�ͨ�����ʡ�װ�����Ҷ�̼��Ϊ_________�������̼���ϵĵ缫��ӦΪ_________________________���ҳ��е�c(NaOH)��c(Na2S)______________(������������������������������С)��
����Ŀ�����в���ǰ36��Ԫ�ص����ʻ�ԭ�ӽṹ���±�
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
R | ��̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2������ |
S | ��������ˮ���ҷ�Ӧ��������Һ�������� |
T | ��̬ԭ��3d�������1������ |
X | �� |
��1��RԪ�صĵ�һ������Ҫ������ͬ�������ڵ�Ԫ�أ�ԭ����________________________________________________________��
��2��SԪ�صĻ��ϼ��Ƿ������ۣ�__________��ԭ����__________________________________�����������Ų�ʽΪ________________________��
��3��TԪ�ص�ԭ��N�ܲ��ϵ�����Ϊ__________����ԭ�ӽṹʾ��ͼΪ__________��
��4��X�ĺ�������Ų�ͼΥ����__________����X���ʡ�������μ����������εȿ����������ȼ��ʱ�����������ɫ�Ĺ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��____________________________________________________________________��
���𰸡� ��ԭ��2p���������������ͣ��ȶ� �� F�ĵ縺�����ֻ�ܵõ��� 2s22p5 2  �������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�����������������RԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ�R��NԪ�أ�SԪ�صĵ�������ˮ���ҷ�Ӧ��������Һ�������ԣ�S��FԪ�أ�TԪ�صĻ�̬ԭ��3d�������1�����ӣ�T��21��Ԫ��Sc�� XԪ�ص�ԭ�Ӻ�����12�����ӣ�X��MgԪ�ء�
�������������Ϸ�������1��R��NԪ������ԭ��2p���������������ͣ��ȶ�,���Ե�һ������Ҫ������ͬ�������ڵ�OԪ����
��2��Ԫ��F�ĵ縺�����ֻ�ܵõ���������FԪ��û�����ۣ�FԪ�ص����������Ų�ʽΪ2s22p5��
��3��Scԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p63d14s2������N�ܲ��ϵ�����Ϊ2����ԭ�ӽṹʾ��ͼΪ ����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
�����͡�������
��������
20
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ��������ˮ���ҷ�Ӧ��������Һ�������� |
X | L��p��������s��������2�� |
Y | ��������Ԫ�صļ������а뾶��С |
Z | L��������δ�ɶԵ��� |
��1��д��Ԫ��X�����ӽṹʾ��ͼ__________��
��2��д��YԪ������������ˮ����ֱ���HCl��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________��_________________________��
��3��д��Z��Y�ĵ����Ų�ʽ______________��________________��
��4��Ԫ��T����Ԫ����ȣ��ǽ����Խ�ǿ����__________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����__________��
A����̬�⻯��Ļӷ��Ժ��ȶ���
B�����ʷ����еļ���
C����Ԫ�صĵ縺��
D�������������
E���⻯����X��H���ļ���(X����T��Cl����Ԫ��)
F������������Ȼ���еĴ�����ʽ
��5��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�صĵ����л�ѧ�������Բ�ͬ���������ֵ��ʵ���__________(��Ԫ�ط���)��������________________________________________________��