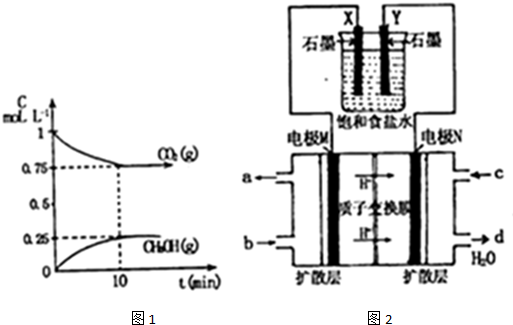
��Ŀ����
9�����з�Ӧ�ķ���ʽ��ȷ���ǣ�������| A�� | AlCl3��Һ�еμ�Ũ��ˮ��������Al3++4NH3•H2O=AlO2-+4NH4++2H2O | |
| B�� | MnO2��Ũ���ᷴӦ��ȡCl2��MnO2+4H++4Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++2Cl2��+2H2O | |
| C�� | С�մ���Һ�м�������ϡ���HCO3-+H+=CO2��+H2O | |
| D�� | ��ⱥ��ʳ��ˮ��������Ӧ��2Cl--2e-=Cl2�� |
���� A��һˮ�ϰ�Ϊ���������Һ��������������
B������غ㲻�غ㣻
C��С�մ�Ϊ̼�����ƣ�̼��������ϡ���ᷴӦ�����Ȼ��ơ�������̼�����ˮ��
D������������ԭ��Ӧ�������ӵõ���������������
��� �⣺A���Ȼ����백ˮ��Ӧ��������������������ȷ�����ӷ���ʽΪ��Al3++3NH3•H2O=Al��OH��3��+3NH4+����A����
B��MnO2��Ũ���ᷴӦ��ȡCl2�����ӷ���ʽ�����������غ㣬��ȷ�����ӷ���ʽΪ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O����B����
C��С�մ���Һ�м�������ϡ���ᣬ��Ӧ�����ӷ���ʽΪ��HCO3-+H+=CO2��+H2O����C��ȷ��
D����ⱥ��ʳ��ˮʱ�����������ӵõ�����������������ȷ�ĵ缫��ӦʽΪ��2H++2e-=H2������D����
��ѡC��
���� ���⿼�������ӷ���ʽ���жϣ���Ŀ�Ѷ��е��⣬ע���������ӷ���ʽ����дԭ����ȷ���ӷ���ʽ�����жϳ��÷������磺��鷴Ӧ��������Ƿ���ȷ���������ʲ���Ƿ���ȷ���������������ʵ���Ҫ������ѧʽ��D����Ҫ��ȷ���ԭ����Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
19�������£����й��ڵ������Һ��˵����ȷ���ǣ�������
| A�� | ��pH=4 CH3COOH��Һ��ˮϡ��10������Һ�и�����Ũ�Ⱦ���С | |
| B�� | �� CH3COOH��Һ�ζ������ʵ���Ũ�ȵ�NaOH��Һ��pH=7��V��CH3COOH��Һ����V��NaOH��Һ�� | |
| C�� | ��0.2 mol/L��������Һ�м�������0.1 mol•L-1NH3•H2O��Һ��c��Cl-��+c��OH-��=c��H+��+c��NH3•H2O�� | |
| D�� | �ں�0.1mol NaHSO4��Һ�У�c��H+��=c��SO42-��+c��OH-�� |
20���������ӷ���ʽ��ȷ���ǣ�������
| A�� | KI��Һ�еμ�ϡ���4H++4I-+O2=2I2+2H2O | |
| B�� | ����SO2ͨ�백ˮ�У�2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| C�� | NaAlO2��Һ�еμӹ������AlO2-+H2O+H+=Al��OH��3�� | |
| D�� | ��ˮ����AgNO3��Һ����������ȫ��Ag++2NH3•H2O=[Ag��NH3��2]++2H2O |
17��������Ҫ���л�����ԭ�ϣ����й��ڱ�������˵����ȷ���ǣ�������
| A�� | ���³�ѹ��Ϊ���� | B�� | �ܷ���������Ӧ | ||
| C�� | ���ܷ���ȡ����Ӧ | D�� | ������ˮ |
4��ȡ������������ɵĻ�������ˮ�г�ַ�Ӧ����ȡ�����������ϴ�Ӹ��������������ԭ�������������36.3g��ȡͬ������ԭ��������ˮ�г�ַ�Ӧ���ټ���������ϡ���ᣬ������ú���ˣ�����Һ���ɣ��õ�FeSO4•7H2O���壬����������ԭ�������������1.35g���������е��������۵����ʵ���֮��Ϊ��������
| A�� | 1��1 | B�� | 2��3 | C�� | 3��5 | D�� | 6��7 |
14�������£����и����������ƶ���Һ��һ���ܴ���������ǣ�������
| A�� | FeCl3��Һ�У�K��CH3OH��Br-��NO3- | |
| B�� | �ڰ�ˮ��Һ�У�Al3+��NO3-��Cl-��Ag+ | |
| C�� | ij���������Һ�У�NH4+��Fe3+��NO3-��Cl- | |
| D�� | ��ʹ�����Ժ�ɫ����Һ��K+��Cr2O72-��CH2CH2OH��SO42- |
1������װ���ܴﵽʵ��Ŀ���ǣ�������
| A�� |  ��ͼ��ʾװ�����ʵ�����ư��� | |
| B�� |  ��ͼ��ʾװ������FeCl3��Һ�Ʊ���ˮFeCl3 | |
| C�� |  ��ͼ��ʾװ�ó�ȥHCl�к��е�����Cl2 | |
| D�� |  ��ͼ��ʾװ�ÿ����ڷ���������̺Ͷ��Ȼ��� |
13��������ʵ����ʵ���ó�����Ӧ������ȷ���ǣ�������
| ʵ����ʵ | ���� | |
| �� | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ | ������һ����SO2 |
| �� | ��ȼ�յ�þ������CO2���ܼ���ȼ�� | ��ԭ�ԣ�Mg��C |
| �� | NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ���� | ���ԣ�HCO3-��Al��OH��3 |
| �� | �����°�����ȼ�������ڷŵ�ʱ����������Ӧ | �ǽ����ԣ�P��N |
| �� | ij��ɫ��Һ�м�������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ���� | ����Һһ����NH4+ |
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢۢ� | D�� | �ۢܢ� |